- 您的位置:
- 标准下载网 >>
- 标准分类 >>
- 商检行业标准(SN) >>
- SN/T 1895-2007 食品中金黄色葡萄球菌的快速计数法 PetrifilmTM测试片法
【商检行业标准(SN)】 食品中金黄色葡萄球菌的快速计数法 PetrifilmTM测试片法
本网站 发布时间:
2024-06-26 17:04:49
- SN/T1895-2007
- 现行
- 点击下载此标准
标准号:
SN/T 1895-2007
标准名称:
食品中金黄色葡萄球菌的快速计数法 PetrifilmTM测试片法
标准类别:
商检行业标准(SN)
标准状态:
现行-
发布日期:
2007-05-23 -
实施日期:
2007-12-01 出版语种:
简体中文下载格式:
.rar.pdf下载大小:
1.67 MB
标准ICS号:
数学、自然科学>>微生物学>>07.100.30中标分类号:
医药、卫生、劳动保护>>卫生>>C53食品卫生
点击下载
标准简介:
标准下载解压密码:www.bzxz.net
SN/T 1895-2007 食品中金黄色葡萄球菌的快速计数法 PetrifilmTM测试片法 SN/T1895-2007
部分标准内容:
中华人民共和国出入境检验检疫行业标准SN/T1895--2007
食品中金黄色葡萄球菌的快速计数法PetrifilmTM测试片法
Rapid enumeration of Staphylococcus aureus in foods-PetrifilmTM staph express count plate method2007-05-23发布
中华人民共和国
数码防伪
宝家质量监督检验检疫总局
2007-12-01实施
SN/T1895—2007
本标准第一法修改采用了美国分析化学协会(AOAC)官方方法2003.07特定预加工食品和加工食品(冷冻干层面、奶油冻、冷冻什锦蔬菜、冷冻洋芋饼和冷冻裹浆蘑菇)中金黄色葡萄球菌的计数PetrifilmTM金黄色葡萄球菌测试片法LAOACOfficialMethodSM2003.07:PetrifilmTMStaphExpressCount PlateMethod for the Enumeration of Staphylococcusaureus in Selected Types of Processed andPrepared Foods (frozen lasagna,custard,frozen mixed vegetables,frozen hash browns,and frozen battercoatedmushrooms)l;AOAC官方方法20o3.08特定乳制品(冰淇淋、原奶、酸奶、奶酪和乳清粉)中金黄色葡萄球菌的计数PetrifilmTM金黄色葡萄球菌测试片法【AOAC?OfficialMethodM2003.08:PetrifilmFM Staph Express Count Plate Method for the Enumeration of Staphylococcus aureus in Selected Dairy Foods. (strawberry ice cream,raw milk,vanilla yogurt,whey powder,and mozzarellacheese)]:AOAC官方方法2003.11特定肉、家禽和海产品(熟制和切割的鸡肉、汉堡、三文鱼和香肠)中金黄色葡萄球菌的计数PetrifilmTM金黄色葡萄球菌测试片法[AOAC?OfficialMethodSM2003.1l:PetrifilmTM Staph Express Count Plate Method for the enumeration of Staphylococcus aureus in Selected Meat,Seafood and Poultry .本标准的附录A是规范性附录。
本标准由国家认证认可监督管理委员会提出并归口。本标准起草单位:中华人民共和国辽宁出人境检验检疫局、中国合格评定国家认可委员会、中华人民共和国内蒙古出人境检验检疫局、中华人民共和国深圳出人境检验检疫局、大连市产品质量检验所、中华人民共和国黑龙江出人境检验检疫局、大连启元科技发展有限公司、3M中国有限公司。本标准主要起草人:卢行安、刘中学、曹际娟、李宏、孙杰、朱海、谢昭聪、陈兆君、刘颜泓、李苏龙、周振亚、陆苏飙。
本标准系首次发布的出人境检验检疫行业标准。ht
1范围
食品中金黄色葡萄球菌的快速计数法PetrifilmTM测试片法
本标准规定了食品中金黄色葡萄球菌的测定(PetrifilmTMP测试片法)。SN/T1895-2007
本标准适用于食品和食物中毒样品中金黄色葡萄球菌的计数,既适用于金黄色葡萄球菌含量较高的食品也适用于金黄色葡萄球菌含量较低而杂菌含量较高的食品。2规范性引用文件
下列文件中的条款通过本标准的引用而成为本标准的条款。凡是注日期的引用文件,其随后所有的修改单(不包括勘误的内容)或修订版均不适用于本标准,然而,鼓励根据本标准达成协议的各方研究是否可使用这些文件的最新版本。凡是不注日期的引用文件,其最新版本适用于本标准。SN0172出口食品中金黄色葡萄球菌检验方法3原理
PetrifilmTM金黄色葡萄球菌测试片(StaphExpressCountPlate,STX)是一种预先制备好的快速检验系统。它含有具有显色功能并经改良的Baird-Parker培养基,对金黄色葡萄球菌具有很强的选择性,并含有冷水可溶性凝胶。测试片上的紫红色菌落为金黄色葡萄球菌。当测试片上出现除紫红色以外的其他任何颜色(如黑色或蓝绿色),则必须使用确认反应片。此确认反应片含有显色剂和脱氧核糖核酸(DNA)。金黄色葡萄球菌产生的脱氧核糖核酸酶(DNase)会和反应片中的显色剂形成粉红色晕圈。4设备和材料
恒温培养箱:36℃士1℃。
4.2均质器(旋刀式或拍击式)或等效的设备。4.3pH计或精密pH试纸。
4.4放大镜或(和)菌落计数器。5培养基和试剂
5.1无菌生理盐水:称取8.5g氯化钠溶于1000mL蒸馏水中,121℃高压灭菌15min。5.21mol/L氢氧化钠(NaOH):称取40g氢氧化钠(NaOH)溶于1000mL蒸馏水中。5.31mol/L盐酸(HCl):移取浓盐酸90mL,用蒸馏水稀释至1000mL。5.4PetrifilmTM金黄色葡萄球菌测试片。5.5PetrifilmTM金黄色葡萄球菌确认反应片。1)为美国3M公司产品的商品标志。ht
SN/T1895—2007
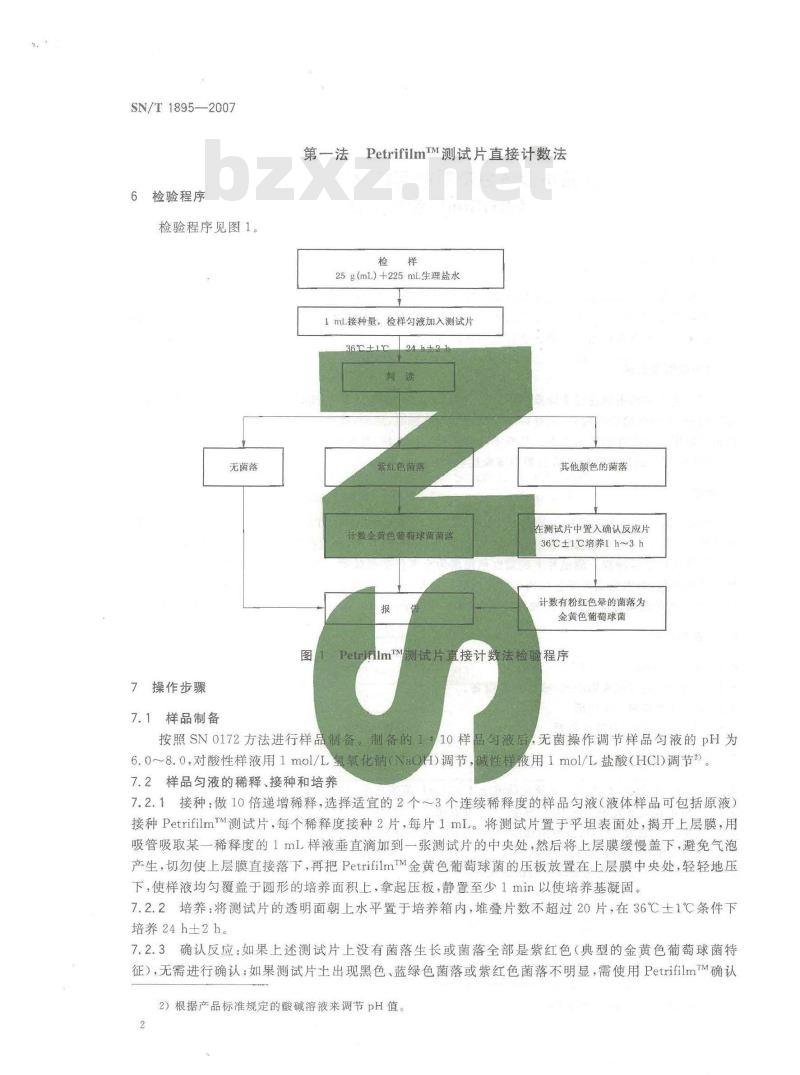
6检验程序
检验程序见图1。
无菌落
7操作步骤
7.1样品制备
第一法
PetrifilmTM测试片直接计数法
25g(mL)+225mL生理盐水
1mL接种量,检样匀液加入测试片荆读
紫红色南酱免费标准下载网bzxz
计数金费色简葡球菌菌菜
其他颜色的菌落
在测试片中置入确认反应片
36℃±1℃培养1h~3h
计数有粉红色晕的菌落为
金黄色葡萄球菌
PetririlmM测试片直接计数法检验程序按照SN0172方法进行样品制备。制备的证10样品液后
,无菌操作调节样品勾液的pH为6.0~8.0,对酸性样液用1mol/L氢氧化钠(NaOH)调节,碱性样夜用1mol/L盐酸(HCI)调节?。7.2样品匀液的稀释、接种和培养7.2.1接种:做10倍递增稀释,选择适宜的2个~~3个连续稀释度的样品匀液(液体样品可包括原液)接种PetrifilmrM测试片,每个稀释度接种2片,每片1mL。将测试片置于平坦表面处,揭开上层膜,用吸管吸取某一稀释度的1mL样液垂直滴加到一张测试片的中央处,然后将上层膜缓慢盖下,避免气泡产生,切勿使上层膜直接落下,再把PetrifilmTM金黄色葡萄球菌的压板放置在上层膜中央处,轻轻地压下,使样液均匀覆盖于圆形的培养面积上,拿起压板,静置至少1min以使培养基凝固。7.2.2培养:将测试片的透明面朝上水平置于培养箱内,堆叠片数不超过20片,在36℃土1℃条件下培养24h±2h。
确认反应:如果上述测试片上没有菌落生长或菌落全部是紫红色(典型的金黄色葡萄球菌特7.2.3
征),无需进行确认,如果测试片土出现黑色,蓝绿色菌落或紫红色菌落不明显,需使用PetrifilmTM确认2)根据产品标准规定的酸碱溶液来调节pH值。htt
反应片作进一步确认。
SN/T1895—2007
将上层膜掀起,将确认反应片置人测试片的培养范围内,再将上层膜放下覆盖在确认反应片上,用手指以滑动的方式轻轻将测试片与确认反应片压紧,包括确认反应片的边缘,此步骤可使测试片与PetrifilmTM确认反应片紧密接触并除去气泡,最后把插人确认反应片的测试片放在36℃士1℃的培养箱内培养1 h~3 h。
8结果计算与报告
8.1判读:紫红色的菌落直接计数为金黄色葡萄球菌需要使用确认反应片作确认时,计数有粉红色晕圈的菌落。没有粉红色晕圈的菌落不是金黄色葡萄球菌,不应被计数。如果整个培养面积呈粉红色而没有明显的晕圈,说明金黄色葡萄球菌大量存在,结果记录为“多不可计”。8.2菌落计数:培养结束后立即计数,可目视或用菌菠计数器来计数,放大镜可辅助计数:选取金黄色葡萄球菌菌落数在15~150之间的测试片,计数菌落数,乘以相对应的稀释倍数报告之,如果所有稀释度测试片上的菌落数都小于15,则计数稀释度最低的测试片上的菌落数乘以稀释倍数报告之,如果所有稀释度的测试片上均无菌落生长,厕以小手1乘以最低稀释倍数报告之;如果最高稀释度的菌落数大于150个时,计数最高稀释度的测试片上的菌落数乘以稀释信数报告之。报告单位以CFU/g(mL)表示。
第二法
9检验程序
检验程序见图2。
Petrifilm
25g(ml
M测试片MPN法
225mL稀释液
1O倍梯度稀择
个适宣稀释度的样晶勾液,吸取1液
每个稀释度样品匀液
各接种3张测试片
24h土
查MPN表
报告结果
直接计数
图2金黄色葡萄球菌PetrifilmrM测试片MPN法检验程序10操作步骤
10.1样品制备
见7.1。
10.2样品匀液的接种
分别在做10倍递增稀释的同时,选择适宜的3个连续稀释度的样品匀液(液体样品可包括原液)3
http
SN/T1895—2007
吸取样品勾液,以1mL接种量加人到3张测试片。每个稀释度接种3张,接种方法见7.2.1。10.3培养和确认
见7.2.2和7.2.3。
10.4判读
金黄色葡萄球菌菌落判读见8.1,如果最低稀释度的3个纸片不都有确认的金黄色葡萄球菌菌落,可根据金黄色葡萄球菌菌落的存在与否,对所有9张测试片进行阳性或阴性的定性报告,而无须计数每张测试片上金黄色葡萄球菌菌落数目。如果最低稀释度的3个测试片上均有确认的金黄色葡萄球菌菌落,可以按照上述的方法对每张测试片进行金黄色葡萄球菌定性报告,也可以采用平板直接计数的方法,计算测试片上的金黄色葡萄球菌菌落数目。
11结果报告
根据金黄色葡萄球菌阳性纸片数,查MPN检索表(见附录A),报告每g(mL)样品中金黄色葡萄球菌的MPN值。如果可以直接计数的结果报告,见8.2。http:
附录A
(规范性附录)
1g(mL)检样中最可能数(MPN)表SN/T1895—2007
1g(mL)检样中最可能数(MPN)表,见表A1。使用九张测试片法,接种量(相当于样品的量)分别为0.1g(mL)0.01g(mL),0.001g(mL)。表A.11g(mL)检样中最可能数(MPN)表阳性纸片数
95%置信区间
阳性纸片数
95%置信区间
注:表内所列检样量如改用0.01g(mL)、0.001g(mL)0.0001g(mL)时,则表内数字应相应增加10倍,其余类推。
ht
SN/T1895—2007
Foreword
The method 1 in this standard refers to A0AcOfficial MethodsM 2003.07:PetrifilmTM Staph ExpressCount Plate Method for the Enumeration of Staphylococcus aureus in Selected Types of Processedand Prepared Foods (frozen lasagna,custard,frozen mixed vegetables,frozen hash browns,andfrozen batter coated mushrooms);AOAc Official MethodsM 2003.08:PetrifilmTM Staph ExpressCount Plate Method for the Enumeration of Staphylococcus aureus in Selected Dairy Foods.(straw-berry ice cream, raw milk,vanilla yogurt,whey powder,and mozzarella cheese); AoAc OfficialMethod sM 2003.11:PetrifilmTM staph Express Count Plate Method for the enumeration of Staphylo-coccusaureus in Selected Meat,Seafaod and PoultryAnnexAis normative.
This standardwas propasedby Certification and AclicofChina(CNCA),andisunderthe jurisalictiontationAdministratorofthepeople'sRepub-ofCNCA.
The standard was drafted by Liaoning Entry-exit Export InspectionChina,China National Accreditat on Service for Conformity Asseand Quarantine Bureau of P.R.sment(CNAS)InnerMongolia
Entry-exit Export Inspection and Quarantine Bureau of P.R.China,Shenzhen Entry-exit ExportInspection and Quarantine Bureau of P R. China Dalian Institute of testing on Product Quality,ofP.R.ChinaDalianNew Eratechctionand Qu
darantine Bureau
Heilongjiang Entry-exit Export Inspegnologydevelopment Itd.,3MChinae
The main drafers of this standard arLuXing
Hai,XieZhaocong,ChenZhaojun,LuYanho
ZhongxueCao Jijuan,LiHong,Sun JieZhuSulong,
Zhou Zhenya and Lu Subiao.
This standard is a professionalI standard of entry-exit inspection and quarantine promulgated for thefirst time.
ht
SN/T1895—2007
RapidenumerationofStaphylococcusaureusinFoodstPetrifilmTM staph express countplate (STX)method1Scope
This standard specifies the rapid enumeration of Staphylococcus aureus in Foods-3M PetrifilmTM 1sStaph Express Count Plate (STX) method.This standard is applicable to the enumeration of the staphylococcus aureus in food and toxicsample. It is applied to not only the sample at high concentration of s.aureus,but also the the sam-pleat low concentration of s.2 Normative reference
The following standard contains provisions whicharo
reference in this text, constitute provi-sions of this standard. For dated referencas, the subsequent anendments (besides the incorrectcontent) or revisions are not applied to this standard. But the person who reach the agreement onthis standard are encouraged to stucgdtionof these references. For undatedreferences the lastest edition of the publication referred to applies.Yof Staphylococcus aureus in food for exportSN 0172 Method for Detectior3Principle
3MTM PetrifilmTM Staph Express CountPlate
contains a cold-water-soluble gelling agent, Theplate is selective and differential far s.aureustisa
hromogenie.
Ise culture medium system whichbdifiedBaird-Parkermedium intheRed-vlolet
colonies on the plate are S. aureus. Ifnon-violet colonies occur such as black or blus-green colonishould be used to identify S. aureusallsuspect
the3MPetrilmStaphExpressDiskfonies.Staph Express Disk contains a dyeand deoxyribonucleic acid (DNA).. S. aureus produces deoxyribonuclease (DNase) and the DNase reacts withthe dyeto formpink zones.4
Equipment andmaterials
Thermostatic incubator:36℃±1℃4.2
Stomacher,blender or equivalent.1) Petrifilm is the trademark of 3M company.ht
SN/T1895-2007
pH meter or precise pH paper.Magnifierand/orcolonycounter5
Medium andagent
Sterile saline solution:Weigh 8.5 g NaCl and dissolve into 1 000 mL distilled water, and thenautoclavefor15minat121℃.
1mol/L NaOH:Weigh 40 gNaOHand then dissolve it into 1000 mL distilled water.5.3
1mol/LHCl:Pipe90ml HCl,and then dilute it into1000mLdistilled water.5.4
PetrifilmTMStaphExpressCountPlate(STX)PetrifilmTM STX Disk.
Method1 3MPetrifilmTMStaphExpressCountPlate(STX)Direct EnumerationMethodFlowChart
The flow chart is showed in figure 1.Sampling
25g(mL)+225 mLBuffer wate
Inoculate1 mL suspensiononto STX36℃±1℃
24h±2h
Interpretation
No suspect
Colony
Only violet red colony
Count all the violet red
colony as s.aureus
Non-violet red colonies
Insert Petrifilm TM STX Disk,and incubate 1-3 hrs
Count all the pink zone as
figure 1-The flow chartof 3MPetrifilmTM Staph ExpressCount Plate(STX)direct enumeration methodht
7Procedure
7.1Samplepreparation
SN/T1895—2007
The sample is prepared in accordance with SN o172. After preparing the 1 :10 dilution,the pH ofthe dilution should be adjusted to 6.0~8.0,1 mol/L NaOH is used to adjust acidic sample,and1 mol/L HCl is used to adjust alkaline sample?,7.2Inoculation and incubationInoculation:For each sample,after preparing the decimal dilutions, select suitalbe 2-37.21
continous dilutions (Fluid sample can be undiluted) to inoculate 1 ml onto each PetrifilmTM STX plate,and two plates for each dilution.Lift top film and inoculate 1mL test suspension onto center of filmbase by pipette.Carefullyand gently roll top film down on inoculum to avoid the gas bubble.Do notlet the top film down directly. Distribute suspension over prescribed growth area with downwardpressure in center of plastic spreader device. Leave plate undisturbed at least 1 min to permit gel tosolidify.
7.2.2Incubation:In incubator,place plates in horizontal position,clear side up,in stacks notexceeding20units.Theincubationconditionis36℃±1℃,and24h±2h.7.2.3Confirm reaction: If there is no growth of any bacteria or all the colonies are violet red (typi-cal characteristic s.aureus),the use of disk is unnecessary; If there is any black or blue-green colo-nies or non-obvious violet red colonies, the 3M PetrifilmTM STx Disk should be used for further con-firmation.
Lift the top film. and then insert the disk into the well of the plate, rejoin the top film and cover thedisk. Use finger to press the plate and disk tightly including the edge of the disk. The air bubblesbetween the 3M PetrifilmTM plate and disk can be removed in this step. Finally put the plate inserteddiskintotheincubatorat36℃±1℃for1h~3h.8
Resultcalculationandreport
8.1 Interpretation:Count the violet red colonies as confirmed S. aureus without disk insert;Whenthe colonies are confirmed by disk,count all the pink zone as S.aureus.The colonies with pink zoneare not S. aureus, and cannot be counted. If the whole inoculated area turn pink and there is noobvious zone,it means that there is great amount of S.aureus, and report the result as\TNTC\(toonumerous to count). Further dilution and retest is needed for more accurate result.8.2Colony calculation:Count the colonies promptly after the incubation period by eyes or stand-ard colony counter. The magnifier-illuminator may also be used to facilitate counting. Select the2)pH can alsobe adjustedaccordingto theproductspecification.食品伙伴
SN/T1895—2007
plate when the colonies are within the range from 15 to 150 to count the colonies, and then multiplythe dilution factor to report; If the number of colonies on all the plates is lower than 15, count thecolonieson theplatewiththelowestdilution,and thenmultiplythe dilutionfactor toreport; If thereis no growth on the plates with all the dilution, then report result as lower than one multiply thelowestdilutionfactor;If thecoloniesontheplatewiththehighestdilution isgreaterthan15o,countthe colonies on plate with the highest dilution, and then multiply the dilution factor for report. Addi-tonallycirculargrowthareais approximately3o cm?.Estimatescanbemade on plates containingmorethan15o colonies by countingthenumberof colonies inoneormorerepresentativesquaresanddetermining the average number per square. Multiply the average numberby 3o to determine totalcountperplate.“cfu/g(ml)\isusedasthereportunit.Method23MPetrifilmTM StaphExpressCountPlate(STX)MPNMethod9Flow chart
The flow chart is showed in figure 2.25gmj2hmldtuuom
ablediutionandinocntateImtofhdilutior
8℃±1
CheckMn Tab
6astxplae
Report
Figure2+-Theflowchartof3MPetrifilmTMStaphExpress countPlate(STX)MPNmethod10
Procedure
Samplepreparation
Refer to 7.1.
ht
小提示:此标准内容仅展示完整标准里的部分截取内容,若需要完整标准请到上方自行免费下载完整标准文档。
食品中金黄色葡萄球菌的快速计数法PetrifilmTM测试片法
Rapid enumeration of Staphylococcus aureus in foods-PetrifilmTM staph express count plate method2007-05-23发布
中华人民共和国
数码防伪
宝家质量监督检验检疫总局
2007-12-01实施
SN/T1895—2007
本标准第一法修改采用了美国分析化学协会(AOAC)官方方法2003.07特定预加工食品和加工食品(冷冻干层面、奶油冻、冷冻什锦蔬菜、冷冻洋芋饼和冷冻裹浆蘑菇)中金黄色葡萄球菌的计数PetrifilmTM金黄色葡萄球菌测试片法LAOACOfficialMethodSM2003.07:PetrifilmTMStaphExpressCount PlateMethod for the Enumeration of Staphylococcusaureus in Selected Types of Processed andPrepared Foods (frozen lasagna,custard,frozen mixed vegetables,frozen hash browns,and frozen battercoatedmushrooms)l;AOAC官方方法20o3.08特定乳制品(冰淇淋、原奶、酸奶、奶酪和乳清粉)中金黄色葡萄球菌的计数PetrifilmTM金黄色葡萄球菌测试片法【AOAC?OfficialMethodM2003.08:PetrifilmFM Staph Express Count Plate Method for the Enumeration of Staphylococcus aureus in Selected Dairy Foods. (strawberry ice cream,raw milk,vanilla yogurt,whey powder,and mozzarellacheese)]:AOAC官方方法2003.11特定肉、家禽和海产品(熟制和切割的鸡肉、汉堡、三文鱼和香肠)中金黄色葡萄球菌的计数PetrifilmTM金黄色葡萄球菌测试片法[AOAC?OfficialMethodSM2003.1l:PetrifilmTM Staph Express Count Plate Method for the enumeration of Staphylococcus aureus in Selected Meat,Seafood and Poultry .本标准的附录A是规范性附录。
本标准由国家认证认可监督管理委员会提出并归口。本标准起草单位:中华人民共和国辽宁出人境检验检疫局、中国合格评定国家认可委员会、中华人民共和国内蒙古出人境检验检疫局、中华人民共和国深圳出人境检验检疫局、大连市产品质量检验所、中华人民共和国黑龙江出人境检验检疫局、大连启元科技发展有限公司、3M中国有限公司。本标准主要起草人:卢行安、刘中学、曹际娟、李宏、孙杰、朱海、谢昭聪、陈兆君、刘颜泓、李苏龙、周振亚、陆苏飙。
本标准系首次发布的出人境检验检疫行业标准。ht
1范围
食品中金黄色葡萄球菌的快速计数法PetrifilmTM测试片法
本标准规定了食品中金黄色葡萄球菌的测定(PetrifilmTMP测试片法)。SN/T1895-2007
本标准适用于食品和食物中毒样品中金黄色葡萄球菌的计数,既适用于金黄色葡萄球菌含量较高的食品也适用于金黄色葡萄球菌含量较低而杂菌含量较高的食品。2规范性引用文件
下列文件中的条款通过本标准的引用而成为本标准的条款。凡是注日期的引用文件,其随后所有的修改单(不包括勘误的内容)或修订版均不适用于本标准,然而,鼓励根据本标准达成协议的各方研究是否可使用这些文件的最新版本。凡是不注日期的引用文件,其最新版本适用于本标准。SN0172出口食品中金黄色葡萄球菌检验方法3原理
PetrifilmTM金黄色葡萄球菌测试片(StaphExpressCountPlate,STX)是一种预先制备好的快速检验系统。它含有具有显色功能并经改良的Baird-Parker培养基,对金黄色葡萄球菌具有很强的选择性,并含有冷水可溶性凝胶。测试片上的紫红色菌落为金黄色葡萄球菌。当测试片上出现除紫红色以外的其他任何颜色(如黑色或蓝绿色),则必须使用确认反应片。此确认反应片含有显色剂和脱氧核糖核酸(DNA)。金黄色葡萄球菌产生的脱氧核糖核酸酶(DNase)会和反应片中的显色剂形成粉红色晕圈。4设备和材料
恒温培养箱:36℃士1℃。
4.2均质器(旋刀式或拍击式)或等效的设备。4.3pH计或精密pH试纸。
4.4放大镜或(和)菌落计数器。5培养基和试剂
5.1无菌生理盐水:称取8.5g氯化钠溶于1000mL蒸馏水中,121℃高压灭菌15min。5.21mol/L氢氧化钠(NaOH):称取40g氢氧化钠(NaOH)溶于1000mL蒸馏水中。5.31mol/L盐酸(HCl):移取浓盐酸90mL,用蒸馏水稀释至1000mL。5.4PetrifilmTM金黄色葡萄球菌测试片。5.5PetrifilmTM金黄色葡萄球菌确认反应片。1)为美国3M公司产品的商品标志。ht
SN/T1895—2007
6检验程序
检验程序见图1。
无菌落
7操作步骤
7.1样品制备
第一法
PetrifilmTM测试片直接计数法
25g(mL)+225mL生理盐水
1mL接种量,检样匀液加入测试片荆读
紫红色南酱免费标准下载网bzxz
计数金费色简葡球菌菌菜
其他颜色的菌落
在测试片中置入确认反应片
36℃±1℃培养1h~3h
计数有粉红色晕的菌落为
金黄色葡萄球菌
PetririlmM测试片直接计数法检验程序按照SN0172方法进行样品制备。制备的证10样品液后
,无菌操作调节样品勾液的pH为6.0~8.0,对酸性样液用1mol/L氢氧化钠(NaOH)调节,碱性样夜用1mol/L盐酸(HCI)调节?。7.2样品匀液的稀释、接种和培养7.2.1接种:做10倍递增稀释,选择适宜的2个~~3个连续稀释度的样品匀液(液体样品可包括原液)接种PetrifilmrM测试片,每个稀释度接种2片,每片1mL。将测试片置于平坦表面处,揭开上层膜,用吸管吸取某一稀释度的1mL样液垂直滴加到一张测试片的中央处,然后将上层膜缓慢盖下,避免气泡产生,切勿使上层膜直接落下,再把PetrifilmTM金黄色葡萄球菌的压板放置在上层膜中央处,轻轻地压下,使样液均匀覆盖于圆形的培养面积上,拿起压板,静置至少1min以使培养基凝固。7.2.2培养:将测试片的透明面朝上水平置于培养箱内,堆叠片数不超过20片,在36℃土1℃条件下培养24h±2h。
确认反应:如果上述测试片上没有菌落生长或菌落全部是紫红色(典型的金黄色葡萄球菌特7.2.3
征),无需进行确认,如果测试片土出现黑色,蓝绿色菌落或紫红色菌落不明显,需使用PetrifilmTM确认2)根据产品标准规定的酸碱溶液来调节pH值。htt
反应片作进一步确认。
SN/T1895—2007
将上层膜掀起,将确认反应片置人测试片的培养范围内,再将上层膜放下覆盖在确认反应片上,用手指以滑动的方式轻轻将测试片与确认反应片压紧,包括确认反应片的边缘,此步骤可使测试片与PetrifilmTM确认反应片紧密接触并除去气泡,最后把插人确认反应片的测试片放在36℃士1℃的培养箱内培养1 h~3 h。
8结果计算与报告
8.1判读:紫红色的菌落直接计数为金黄色葡萄球菌需要使用确认反应片作确认时,计数有粉红色晕圈的菌落。没有粉红色晕圈的菌落不是金黄色葡萄球菌,不应被计数。如果整个培养面积呈粉红色而没有明显的晕圈,说明金黄色葡萄球菌大量存在,结果记录为“多不可计”。8.2菌落计数:培养结束后立即计数,可目视或用菌菠计数器来计数,放大镜可辅助计数:选取金黄色葡萄球菌菌落数在15~150之间的测试片,计数菌落数,乘以相对应的稀释倍数报告之,如果所有稀释度测试片上的菌落数都小于15,则计数稀释度最低的测试片上的菌落数乘以稀释倍数报告之,如果所有稀释度的测试片上均无菌落生长,厕以小手1乘以最低稀释倍数报告之;如果最高稀释度的菌落数大于150个时,计数最高稀释度的测试片上的菌落数乘以稀释信数报告之。报告单位以CFU/g(mL)表示。
第二法
9检验程序
检验程序见图2。
Petrifilm
25g(ml
M测试片MPN法
225mL稀释液
1O倍梯度稀择
个适宣稀释度的样晶勾液,吸取1液
每个稀释度样品匀液
各接种3张测试片
24h土
查MPN表
报告结果
直接计数
图2金黄色葡萄球菌PetrifilmrM测试片MPN法检验程序10操作步骤
10.1样品制备
见7.1。
10.2样品匀液的接种
分别在做10倍递增稀释的同时,选择适宜的3个连续稀释度的样品匀液(液体样品可包括原液)3
http
SN/T1895—2007
吸取样品勾液,以1mL接种量加人到3张测试片。每个稀释度接种3张,接种方法见7.2.1。10.3培养和确认
见7.2.2和7.2.3。
10.4判读
金黄色葡萄球菌菌落判读见8.1,如果最低稀释度的3个纸片不都有确认的金黄色葡萄球菌菌落,可根据金黄色葡萄球菌菌落的存在与否,对所有9张测试片进行阳性或阴性的定性报告,而无须计数每张测试片上金黄色葡萄球菌菌落数目。如果最低稀释度的3个测试片上均有确认的金黄色葡萄球菌菌落,可以按照上述的方法对每张测试片进行金黄色葡萄球菌定性报告,也可以采用平板直接计数的方法,计算测试片上的金黄色葡萄球菌菌落数目。
11结果报告
根据金黄色葡萄球菌阳性纸片数,查MPN检索表(见附录A),报告每g(mL)样品中金黄色葡萄球菌的MPN值。如果可以直接计数的结果报告,见8.2。http:
附录A
(规范性附录)
1g(mL)检样中最可能数(MPN)表SN/T1895—2007
1g(mL)检样中最可能数(MPN)表,见表A1。使用九张测试片法,接种量(相当于样品的量)分别为0.1g(mL)0.01g(mL),0.001g(mL)。表A.11g(mL)检样中最可能数(MPN)表阳性纸片数
95%置信区间
阳性纸片数
95%置信区间
注:表内所列检样量如改用0.01g(mL)、0.001g(mL)0.0001g(mL)时,则表内数字应相应增加10倍,其余类推。
ht
SN/T1895—2007
Foreword
The method 1 in this standard refers to A0AcOfficial MethodsM 2003.07:PetrifilmTM Staph ExpressCount Plate Method for the Enumeration of Staphylococcus aureus in Selected Types of Processedand Prepared Foods (frozen lasagna,custard,frozen mixed vegetables,frozen hash browns,andfrozen batter coated mushrooms);AOAc Official MethodsM 2003.08:PetrifilmTM Staph ExpressCount Plate Method for the Enumeration of Staphylococcus aureus in Selected Dairy Foods.(straw-berry ice cream, raw milk,vanilla yogurt,whey powder,and mozzarella cheese); AoAc OfficialMethod sM 2003.11:PetrifilmTM staph Express Count Plate Method for the enumeration of Staphylo-coccusaureus in Selected Meat,Seafaod and PoultryAnnexAis normative.
This standardwas propasedby Certification and AclicofChina(CNCA),andisunderthe jurisalictiontationAdministratorofthepeople'sRepub-ofCNCA.
The standard was drafted by Liaoning Entry-exit Export InspectionChina,China National Accreditat on Service for Conformity Asseand Quarantine Bureau of P.R.sment(CNAS)InnerMongolia
Entry-exit Export Inspection and Quarantine Bureau of P.R.China,Shenzhen Entry-exit ExportInspection and Quarantine Bureau of P R. China Dalian Institute of testing on Product Quality,ofP.R.ChinaDalianNew Eratechctionand Qu
darantine Bureau
Heilongjiang Entry-exit Export Inspegnologydevelopment Itd.,3MChinae
The main drafers of this standard arLuXing
Hai,XieZhaocong,ChenZhaojun,LuYanho
ZhongxueCao Jijuan,LiHong,Sun JieZhuSulong,
Zhou Zhenya and Lu Subiao.
This standard is a professionalI standard of entry-exit inspection and quarantine promulgated for thefirst time.
ht
SN/T1895—2007
RapidenumerationofStaphylococcusaureusinFoodstPetrifilmTM staph express countplate (STX)method1Scope
This standard specifies the rapid enumeration of Staphylococcus aureus in Foods-3M PetrifilmTM 1sStaph Express Count Plate (STX) method.This standard is applicable to the enumeration of the staphylococcus aureus in food and toxicsample. It is applied to not only the sample at high concentration of s.aureus,but also the the sam-pleat low concentration of s.2 Normative reference
The following standard contains provisions whicharo
reference in this text, constitute provi-sions of this standard. For dated referencas, the subsequent anendments (besides the incorrectcontent) or revisions are not applied to this standard. But the person who reach the agreement onthis standard are encouraged to stucgdtionof these references. For undatedreferences the lastest edition of the publication referred to applies.Yof Staphylococcus aureus in food for exportSN 0172 Method for Detectior3Principle
3MTM PetrifilmTM Staph Express CountPlate
contains a cold-water-soluble gelling agent, Theplate is selective and differential far s.aureustisa
hromogenie.
Ise culture medium system whichbdifiedBaird-Parkermedium intheRed-vlolet
colonies on the plate are S. aureus. Ifnon-violet colonies occur such as black or blus-green colonishould be used to identify S. aureusallsuspect
the3MPetrilmStaphExpressDiskfonies.Staph Express Disk contains a dyeand deoxyribonucleic acid (DNA).. S. aureus produces deoxyribonuclease (DNase) and the DNase reacts withthe dyeto formpink zones.4
Equipment andmaterials
Thermostatic incubator:36℃±1℃4.2
Stomacher,blender or equivalent.1) Petrifilm is the trademark of 3M company.ht
SN/T1895-2007
pH meter or precise pH paper.Magnifierand/orcolonycounter5
Medium andagent
Sterile saline solution:Weigh 8.5 g NaCl and dissolve into 1 000 mL distilled water, and thenautoclavefor15minat121℃.
1mol/L NaOH:Weigh 40 gNaOHand then dissolve it into 1000 mL distilled water.5.3
1mol/LHCl:Pipe90ml HCl,and then dilute it into1000mLdistilled water.5.4
PetrifilmTMStaphExpressCountPlate(STX)PetrifilmTM STX Disk.
Method1 3MPetrifilmTMStaphExpressCountPlate(STX)Direct EnumerationMethodFlowChart
The flow chart is showed in figure 1.Sampling
25g(mL)+225 mLBuffer wate
Inoculate1 mL suspensiononto STX36℃±1℃
24h±2h
Interpretation
No suspect
Colony
Only violet red colony
Count all the violet red
colony as s.aureus
Non-violet red colonies
Insert Petrifilm TM STX Disk,and incubate 1-3 hrs
Count all the pink zone as
figure 1-The flow chartof 3MPetrifilmTM Staph ExpressCount Plate(STX)direct enumeration methodht
7Procedure
7.1Samplepreparation
SN/T1895—2007
The sample is prepared in accordance with SN o172. After preparing the 1 :10 dilution,the pH ofthe dilution should be adjusted to 6.0~8.0,1 mol/L NaOH is used to adjust acidic sample,and1 mol/L HCl is used to adjust alkaline sample?,7.2Inoculation and incubationInoculation:For each sample,after preparing the decimal dilutions, select suitalbe 2-37.21
continous dilutions (Fluid sample can be undiluted) to inoculate 1 ml onto each PetrifilmTM STX plate,and two plates for each dilution.Lift top film and inoculate 1mL test suspension onto center of filmbase by pipette.Carefullyand gently roll top film down on inoculum to avoid the gas bubble.Do notlet the top film down directly. Distribute suspension over prescribed growth area with downwardpressure in center of plastic spreader device. Leave plate undisturbed at least 1 min to permit gel tosolidify.
7.2.2Incubation:In incubator,place plates in horizontal position,clear side up,in stacks notexceeding20units.Theincubationconditionis36℃±1℃,and24h±2h.7.2.3Confirm reaction: If there is no growth of any bacteria or all the colonies are violet red (typi-cal characteristic s.aureus),the use of disk is unnecessary; If there is any black or blue-green colo-nies or non-obvious violet red colonies, the 3M PetrifilmTM STx Disk should be used for further con-firmation.
Lift the top film. and then insert the disk into the well of the plate, rejoin the top film and cover thedisk. Use finger to press the plate and disk tightly including the edge of the disk. The air bubblesbetween the 3M PetrifilmTM plate and disk can be removed in this step. Finally put the plate inserteddiskintotheincubatorat36℃±1℃for1h~3h.8
Resultcalculationandreport
8.1 Interpretation:Count the violet red colonies as confirmed S. aureus without disk insert;Whenthe colonies are confirmed by disk,count all the pink zone as S.aureus.The colonies with pink zoneare not S. aureus, and cannot be counted. If the whole inoculated area turn pink and there is noobvious zone,it means that there is great amount of S.aureus, and report the result as\TNTC\(toonumerous to count). Further dilution and retest is needed for more accurate result.8.2Colony calculation:Count the colonies promptly after the incubation period by eyes or stand-ard colony counter. The magnifier-illuminator may also be used to facilitate counting. Select the2)pH can alsobe adjustedaccordingto theproductspecification.食品伙伴
SN/T1895—2007
plate when the colonies are within the range from 15 to 150 to count the colonies, and then multiplythe dilution factor to report; If the number of colonies on all the plates is lower than 15, count thecolonieson theplatewiththelowestdilution,and thenmultiplythe dilutionfactor toreport; If thereis no growth on the plates with all the dilution, then report result as lower than one multiply thelowestdilutionfactor;If thecoloniesontheplatewiththehighestdilution isgreaterthan15o,countthe colonies on plate with the highest dilution, and then multiply the dilution factor for report. Addi-tonallycirculargrowthareais approximately3o cm?.Estimatescanbemade on plates containingmorethan15o colonies by countingthenumberof colonies inoneormorerepresentativesquaresanddetermining the average number per square. Multiply the average numberby 3o to determine totalcountperplate.“cfu/g(ml)\isusedasthereportunit.Method23MPetrifilmTM StaphExpressCountPlate(STX)MPNMethod9Flow chart
The flow chart is showed in figure 2.25gmj2hmldtuuom
ablediutionandinocntateImtofhdilutior
8℃±1
CheckMn Tab
6astxplae
Report
Figure2+-Theflowchartof3MPetrifilmTMStaphExpress countPlate(STX)MPNmethod10
Procedure
Samplepreparation
Refer to 7.1.
ht
小提示:此标准内容仅展示完整标准里的部分截取内容,若需要完整标准请到上方自行免费下载完整标准文档。
标准图片预览:




- 热门标准
- 商检行业标准(SN)
- SN/T4322-2015 食品接触材料高分子材料双酚A残留量的测定酶联免疫法
- SN/T3987-2014 牛海绵状脑病特殊风险物质实时荧光RT-PCR检测方法
- SN/T3756-2013 玉米细菌性枯萎病菌检疫鉴定方法PCR方法
- SN/T0944-2000 出口冻虾及虾制品中吲哚检验方法比色法
- SN0425-1995 出口冻水煮牛肉检验规程
- SN0667-1997 出口肉及肉制品中丙酸睾丸酮残留量检验方法放射免疫法
- SN/T1891.10-2007 进出口微波食品包装容器及包装材料卫生标准 第10部分:聚苯乙烯树脂
- SN/T4773-2017 玩具材料中N-亚硝胺和N-亚硝基化合物迁移量的测定高效液相色谱-串联质谱法
- SN/T4538.1-2016 商品化试剂盒检测方法三聚氰胺方法一
- SN/T1443.2-2004 食品安全管理体系 审核指南
- SN/T1283-2003 国境口岸结核病监测规程
- SN/T0916-2000 进出口茶叶磨碎试样干物质含量的测定方法
- SN/T0917-2000 进出口茶叶品质感官审评方法
- SN/T0958-2000 进出口冻蚕蛹检验规程
- SN/T0777-1999 出口冻鱵鱼片检验规程
- 行业新闻
请牢记:“bzxz.net”即是“标准下载”四个汉字汉语拼音首字母与国际顶级域名“.net”的组合。 ©2025 标准下载网 www.bzxz.net 本站邮件:bzxznet@163.com
网站备案号:湘ICP备2025141790号-2
网站备案号:湘ICP备2025141790号-2