- 您的位置:
- 标准下载网 >>
- 标准分类 >>
- 商检行业标准(SN) >>
- SN/T 1957-2007 进出口中药材及其制品中五氯硝基苯残留量检测方法 气相色谱-质谱法
【商检行业标准(SN)】 进出口中药材及其制品中五氯硝基苯残留量检测方法 气相色谱-质谱法
本网站 发布时间:
2024-06-29 00:46:32
- SN/T1957-2007
- 现行
- 点击下载此标准
标准号:
SN/T 1957-2007
标准名称:
进出口中药材及其制品中五氯硝基苯残留量检测方法 气相色谱-质谱法
标准类别:
商检行业标准(SN)
标准状态:
现行-
发布日期:
2007-08-06 -
实施日期:
2008-03-01 出版语种:
简体中文下载格式:
.rar.pdf下载大小:
276.50 KB
点击下载
标准简介:
标准下载解压密码:www.bzxz.net
SN/T 1957-2007 进出口中药材及其制品中五氯硝基苯残留量检测方法 气相色谱-质谱法 SN/T1957-2007
部分标准内容:
中华人民共和国出入境检验检疫行业标准SN/T 1957-2007
进出口中药材及其制品中五氯硝基苯残留量检测方法
气相色谱-质谱法
Determination of quintozene residues in medicinal plant andtheir products for import and export-GC-MS method2007-08-06发布
中华人民共和国
数扭防病
国家质量监督检验检疫总局
2008-03-01实施
本标难的附录A为资料性附录。
本标推由国家认证认可监督管理委员会捉出并归口。本标准起草单位:中华人民共和国天津出人境检验检疫局。木标雄主要起草人:林安清、许泓、张冕、占现、何佳、张骏、吴延晖、肖亚兵。本标准系首欲发布的检验检疫行业标推。SN/T1957--2007
1范围
进出口中药材及其制品中五氯硝基萃残留量检测方法气相色谱-质谱法本标准规定了中药材及共制品中五氯硝基苯残留量气相色谱-质谱检测方法。本标证适用于人参、人参口股液中五氧硝基萃残留量的测定。2方法提要
SN/T 1957—2007
试样用正已烷-丙酮混合溶液提取,经浓统酸磺化,气相色谱-质谱法测定,外标法定量。3试剂和材料
除另有规定外,试剂均为分析纯,水为一·级水。3.1正已炕:色谱纯。
3.2丙酮:色谱纯。
3.3浓硫酸:优级纯。
3.4无水硫酸钠:于650℃灼烧4h.置于于燥器中备用。3.5硫酸钠溶液:10/1.。
3.6五氯硝基苯(Quintozene:(:Cl,NO:,c:AS;82-68-8)标雅品:纯度大于等于 99%。3.7氯硝基苯标准溶液:准确称取适量的五氯硝基苯标准品,用正已烧配制成浓度为100Ag/inL标推诺备液。根据需要用正已烷稀释成适当浓度的标准工作落液。于0℃~~4℃冰中保存。3. 8无水硫酸钠样:80 mm×10 mm内径)筒形漏斗,底部垫约5 mm高脱脂棉,再装10 g无水硫酸钠(3.4).
4仪器和设备
4,1和谱-质谱仪,配电子轰击源(EI)。4.2竭旋混合器。
4.3高速均质器:21 000r/min。4.4离心机:3000r/min
4.5旋转蒸发器。
4.6药材粉碎机。
5 样品制备与保存
取有代表性人参样品约500 多,充分粉碎后过2.0 mm 筛。攻有代表性样品人参口服液约500 名,混句,装人洁净容器内.密封并标阴标记,于4℃冷藏保存。在拍样和制样的操作过程中,应防止样品受到泻染或发生五氛硝基苯残留物含量的变化。6测定步骤
6.1提取
6.1.1人参样品
称取5g(精确至0.01g)试拌于100 mL离心管中,加人50 L丙酮十正已烷(2十8,体积比)溶液,1
SN/T 1957—2007
于高速均质器11000r/min均质5min,3000r/min离心3min,过滤至150mL浓缩瓶中.重复上述操作一次,合并提取液,于50℃水浴旋转蒸发至约50mL.转移至150mL分液漏斗中。6.1.2人参口服液样品
称取5g精确至0.01g)试样于100ml.离心管中,加人50ml.两酮-1-正已烷(2+8.体积比)提取液,涡旋混合5min,转移至500mL分液谣斗中。加人300ml.水,振摇,静置分层,弃去水相。再加人300ml.水,重复上述操作一次。6.2净化
在上述分液斗中加入10ml.浓硫酸(3.3),轻轻标摇0.5min后,静置分层,弃去下层酸液。再重复净化3次~4次(净化至下层酸液呈无色)。再用2×100mL,硫酸钠游液(3.5)洗涤两次,静置分层后,充去水相。将净化液通过无水硫酸钠柱(3.8),用10mL正己烷洗涤光水硫酸钠柱,收集正已烷至依缩瓶中,于50℃水浴旋转蒸发至工10mL正已烷游解残渣+供气相色谱·质谱仪测定。6.3测定
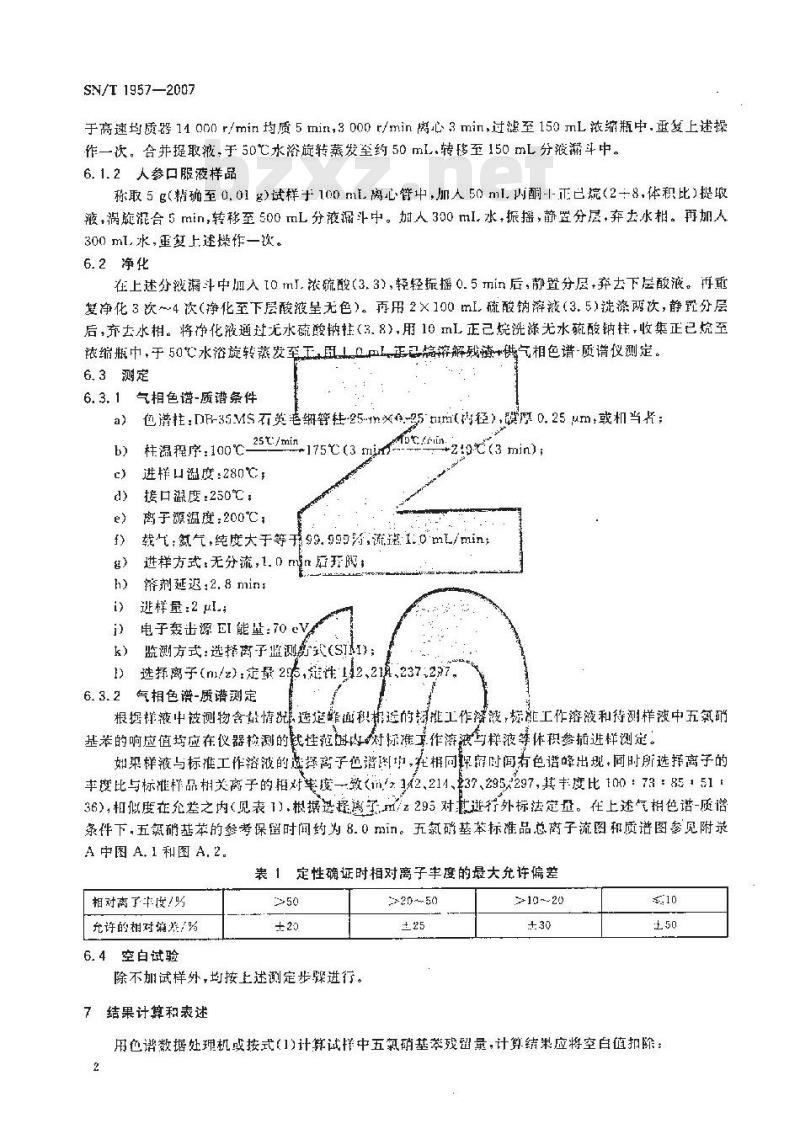
6.3.1气相色谱-质谱条件
包谱柱:DF-35Ms石英港纸警鞋5-m以-g5tImt传径>,膜厚0.25μm或机当片;a)
25t:/min
柱温程序:100℃-
-175C(3 iim
进样口温度:280℃
接口温度:250℃,
离子源温度:200℃;
c(3 min) :
载氢气,纯度大于等于9.99为流1.0mL/ming)进样方式:无分流,1.0mm后开:矫剂延迟:2.8min
逃样量:2
电子轰击源EI能量:70e
监测方式:选择离子监测款式(STAI):k)
1)选择离子(m/2)定量2%,短性142、2真237:2%76.3.2气相色谱-质谱测定
根据样液中被测物含盐情况遥定峰面积棉运的运推工作潜没,标能工作溶液和待测样液中五筑硝基苯的响应值均应在仪器检测的我性范海内对标准工作游液与样液等体积参插进样测定。如架样液与标准工作溶液的趣择离子色谱图功,在相同探留时间有色谱峰出现,同时所选择离子的丰度比与标准样品相关离子的拍对率度一致(mz142、214、毫37、295元297,其丰度比100:73:85:51!36),相似度在允差之内(见表1).根据送凝离子m/z295对就进行外标法定量。在上述气相色谱-质谱条件下,五氮硝基苯的参考保留时间约为8.0min。五氯硝基苯标准品总离子流图和质谱图参见附录A中图A,1和图 A.2.
表1定性确证时相对离子度的最大充许偏差相对离了度/§
允许的相对确差/%
6. 4 空白试验
除不加试样外,均按上述测定步骤进行。7结果计算和表述
>10~-20
用色谱数据处理机或按式(1)计算试样中五氧硝基举残留量,计算结果应将空白值扣除:2
式中:
试样中五氧硝基苯的残留量,单位为毫克每干克(mg/kg):样被中五氧硝基苯的峰面积;
标推工作被中五氣硝基苯的峰面积:标准工作溶液中五氮硝基苯的度,单位为微克持毫升(rg/mL);样被最终定容体积,单位为升(mI):最终样液所代表的试样量,单位为克(g)。8方法的测定低限.回收率
8.1测定低限
本方法测定低限为:0.001mgkg.8.2回收率
本方法添加浓度范围及收率见装表2样品的添加浓度及回收率的实验数据样品
添加依度/(μg/kg)
回收率范}
25:- 954 0
20: 091, 0
25,c~1c0, 0
添加浓度/(rg/kg)
SN/r1957-2007
回收率范围/%
65. 0--97, 5
88,0--97.0
90. 3-- 97. 0
SN/T 1957-2007
附录A
(资料性附录)
五氮硝基苯标准品气相色谱-质图Maui
RT:2.76-12.94
4. M4 n Fr6 Dee. 527. 12
data 0107
022511155
五氨硝基苯标准品总离子流图(IIC)pn1_07U20152503F1034 RT:B.02 AV:1 SB:81B R.21 1D. 91,.:15-6.77 NI.12. 97E6T: (0,0]+= E2det-6D, 0 Full ms[85. 00-31 , 0]100
BIOEEEE
15.毫47.0
.182.8 207.8
五氛硝基苯标准品质谱图
.250. 8
462.8266.8
Foreword
Annex A of this standard is an informative annex.SN/I1957--2007
This standard was proposed by and is under the charge of China National Regulatory Commission forcertificationandAccreditation.This standard was drafted by tianjin entry-exit inspectian and quarantine bureau of the Peaple's re-pubic of China.
The main drafters of this standard are Lin Anging,Xu Hong,Zhang Man,Gu Long,He Jia,Zhang Jun,Wu Yanhui and Xiao Yabing.
This standard is a professional standard promulgated for the first time.SN/T1957—2007
Determination of quintozene resicues in medicinal plant andtheir products for import and export -Gc-Ms methodScope
This standard specifies the methods of determination by Gc-Ms of quintaze residue in medicinalplants and theirs products.
This standard js applicable to the peterminstion of quintoze residue(n panax,gen-seng.
2Principle method of determinationThe Quintozene residue were extractedrwith'acetong-n-hexane,sulfonaped by Oil of vitriol,determi-nated by GC-Ms. Calculated by conyparing peak at.3 Reagents and materials
Unless otherwise specified,all reagentswater.
n-Hexane; HPLC grade.
Acetone: HPLC grade.
Oil of vitriol,Guaranteed r
he sanple.withrcorresponding standard peak area.: :
hofa be anatyticaifx pure. \Water\is the first-degreeAnhydrous sodium sulfate:ignite at sso'c for 4 h,antr'store in air-tight container.Anhydrous sodium sulfate solution:40 g/LQuintozenestandard(C,Cl,NO2,CAS-No82-68-8).Purity99%Quintozene standard solution:Weigh appropriate quintozene,dissolve in n-Hexane and preparea solution of 10o μg/mL as standard stock solution. Dilute the quintozene standard stock solution tothe required concentration as the standard working sotution with n-Hexane. It should be stored in re-frigeratory at o'℃ ~4'℃.
SN/T 1957—2007
3, 8 Anhydrous sodium sulfate colurnn:80 mm × 40 mm(i. d. >funnel, filled with 10 anhydrous so-dium sulfate(3. 4) upon 5 mm.Apparatus and equipment
GC-MS: Equipped with El.
Minishaker
Homogenizer: 24 000 r/mil
Vortex mixer: 3 Do0 r/mir
Rotation evaporator.
Puiverizerbzxz.net
5 Sample preparation and storage Reduce the sample of panax to ca 500 g, churn up, let-pass- through a 2, 0 mm sieve, Reduce the sam-pleof gen-sengtoce5oog.Mixthoroughly,helow 4℃
Place in clean
containers, seal and label, storedIn the course ot sample preparafion, psecautidn should be taken to aviod contamination or any fac-tors which may cause the change Df qtintozene residie conteht.6 Procedure of determination6.1Extraction
6. 1.1 Panax sample
Weigh 5 gt accurated to 0. 0t g) of the test sanmple into 100 mL centrifuge tuba, adding 50 mL ace-tone-n-hexane (2 +B, V/ V), homogenize for 5 min under 14 0o0 r/min, centrifuge for 3 min under3 000 r/min. The extraction liquid was filterated to 150 ml erlenrneyer flake, ther repeated aboveaperation. Combine the extraction liquid, rotary evaporate the extraction liquid at 5o'C to almost50 ml. Transfer above solution to 150 mL separator funinel.SN/T 1957-2007
6. 1.2 Gen-seng sample
Weigh 5 g(accurated to 0.01 g) of the test sample into 100 mL centrifuge tube. adding 50 mL ace.tona-n-hexane.2+ 8, V/ V),minishaking 5 rnin, Transfer above solution to 500 mL separator funnel.adding 3o0 mL water, shaking.iet it stand for separately comperately. Abandon the awater phase.Adding 3oo mL water ,then repeated above operation.6.2Clean up
Add10m oil af vitriol (3.3),shaking lightly for 0,5min,Let it stand for separately comperatelyabandon the acid phase, Repeat above cleaning three or four times(until the acid phase show achro-maticity), Add 2 × 100 mL anhydrous sodizirn suifate solution (3. 5). let it stand for separately comp-erately, abandon the water phase. Pass this liquid through the column of anhydrous sodium suifate(3, 8) to another 150 mL erlenmeyer flake. Rinsc the cloumn of anhydrous sodium sulfate three timeswith 10 ml n-Hexan. Collect n-Hexane phase,rotary evaporate the extraction liquid at 5o' to dry.Make up to 1. 0 mL with n-hexane for GC-MS determination.6.3Determination
6. 3. 1Gc-Ms operating conditionsColumn; DB-35Ms 25 m × o, 25 mmci, d. >. film thickness 0. 25 μm ar equivalent+a)
10c/min-240c(3min));
Column temperature: t0a/75℃(3 min)b)
Injection port temperature: 28o'℃ :d)Interface tempcrature:250'C;e) lon soutce temperature:200' :f)Carrier gas:Helium,purity ≥99. 999%,flow rate;1.0 mL/min?g) Injection mode:Splitless, open the valve after 1. 0 min;Solvent protection delay:2. 8 minth)
i)Injection volumn:2 μL;
j)Jonizatian energY:70 evi
Detectian mode:SiM:
I)Sclected ions(m/z):determinded by295.confirmed by 142.214.237.297.6.3.2Gc-MS determination
SV/T 1957—2007
According to the approximate concentration of quintozene residuces in sample solution, select thestandard working salution with similar peak area to that af the sampla solution. The standard work-ing solutiori should be randomly injected in between the injaction of sarnple solution of equal volumn.if there is a peak appeared at the same retenticn time for both of the sample solution and standardworking solution in the mass spectrogram figure. The accordance between the retention time of themeasured sample solution and the timc of standard,the appearance of all selected ians in the chro-matogram of the sarnple while background is deducted. the consistency between the abundance ratioof the selected ions from the sample and ions from standardGC-MSt relative), %
6. 4 Blank test
20~50
Perfore the blank test with the same procederes as that described in the method of determination butwithout addition of test sample.7 Calculation and expression of the resuitsThe calculation ofX -the residue of quintozene,ing, kg:X-A:cs- V
A-the peak area of cuintozene of the sample solution:As--the peak area of quintozene the Standard working solution;Cs-the coricentration of quintozene in the Standard warking solution, μg/mL;V-the final volume of the sampie solution.ml:.(1)
SN/T 1957-2007
m-the massof test samplein the final solution,g.8Limit of determination and recovery8. 1Limit of determination
The limit of determination of this method is 0, 004 mg/kg.B.2Recovery
The range of fortification and recovery ot this method is shown in table 2Table 2The fortifying concenfratian of chiorfenapyr in samples and its corresponding recoveriesSampte
Fortify cncentration/
<μg/kg)
Retovery/%
75. 0~25, 0
80. D --94. 0
-85:0~100.0
Sampla
gen-seng
Fortlfy cncantration/
《μg/kg)
Recoverv/%
85. 0 ~97. 5
88.0~-97.0
90. 3~ 97. 0
小提示:此标准内容仅展示完整标准里的部分截取内容,若需要完整标准请到上方自行免费下载完整标准文档。
进出口中药材及其制品中五氯硝基苯残留量检测方法
气相色谱-质谱法
Determination of quintozene residues in medicinal plant andtheir products for import and export-GC-MS method2007-08-06发布
中华人民共和国
数扭防病
国家质量监督检验检疫总局
2008-03-01实施
本标难的附录A为资料性附录。
本标推由国家认证认可监督管理委员会捉出并归口。本标准起草单位:中华人民共和国天津出人境检验检疫局。木标雄主要起草人:林安清、许泓、张冕、占现、何佳、张骏、吴延晖、肖亚兵。本标准系首欲发布的检验检疫行业标推。SN/T1957--2007
1范围
进出口中药材及其制品中五氯硝基萃残留量检测方法气相色谱-质谱法本标准规定了中药材及共制品中五氯硝基苯残留量气相色谱-质谱检测方法。本标证适用于人参、人参口股液中五氧硝基萃残留量的测定。2方法提要
SN/T 1957—2007
试样用正已烷-丙酮混合溶液提取,经浓统酸磺化,气相色谱-质谱法测定,外标法定量。3试剂和材料
除另有规定外,试剂均为分析纯,水为一·级水。3.1正已炕:色谱纯。
3.2丙酮:色谱纯。
3.3浓硫酸:优级纯。
3.4无水硫酸钠:于650℃灼烧4h.置于于燥器中备用。3.5硫酸钠溶液:10/1.。
3.6五氯硝基苯(Quintozene:(:Cl,NO:,c:AS;82-68-8)标雅品:纯度大于等于 99%。3.7氯硝基苯标准溶液:准确称取适量的五氯硝基苯标准品,用正已烧配制成浓度为100Ag/inL标推诺备液。根据需要用正已烷稀释成适当浓度的标准工作落液。于0℃~~4℃冰中保存。3. 8无水硫酸钠样:80 mm×10 mm内径)筒形漏斗,底部垫约5 mm高脱脂棉,再装10 g无水硫酸钠(3.4).
4仪器和设备
4,1和谱-质谱仪,配电子轰击源(EI)。4.2竭旋混合器。
4.3高速均质器:21 000r/min。4.4离心机:3000r/min
4.5旋转蒸发器。
4.6药材粉碎机。
5 样品制备与保存
取有代表性人参样品约500 多,充分粉碎后过2.0 mm 筛。攻有代表性样品人参口服液约500 名,混句,装人洁净容器内.密封并标阴标记,于4℃冷藏保存。在拍样和制样的操作过程中,应防止样品受到泻染或发生五氛硝基苯残留物含量的变化。6测定步骤
6.1提取
6.1.1人参样品
称取5g(精确至0.01g)试拌于100 mL离心管中,加人50 L丙酮十正已烷(2十8,体积比)溶液,1
SN/T 1957—2007
于高速均质器11000r/min均质5min,3000r/min离心3min,过滤至150mL浓缩瓶中.重复上述操作一次,合并提取液,于50℃水浴旋转蒸发至约50mL.转移至150mL分液漏斗中。6.1.2人参口服液样品
称取5g精确至0.01g)试样于100ml.离心管中,加人50ml.两酮-1-正已烷(2+8.体积比)提取液,涡旋混合5min,转移至500mL分液谣斗中。加人300ml.水,振摇,静置分层,弃去水相。再加人300ml.水,重复上述操作一次。6.2净化
在上述分液斗中加入10ml.浓硫酸(3.3),轻轻标摇0.5min后,静置分层,弃去下层酸液。再重复净化3次~4次(净化至下层酸液呈无色)。再用2×100mL,硫酸钠游液(3.5)洗涤两次,静置分层后,充去水相。将净化液通过无水硫酸钠柱(3.8),用10mL正己烷洗涤光水硫酸钠柱,收集正已烷至依缩瓶中,于50℃水浴旋转蒸发至工10mL正已烷游解残渣+供气相色谱·质谱仪测定。6.3测定
6.3.1气相色谱-质谱条件
包谱柱:DF-35Ms石英港纸警鞋5-m以-g5tImt传径>,膜厚0.25μm或机当片;a)
25t:/min
柱温程序:100℃-
-175C(3 iim
进样口温度:280℃
接口温度:250℃,
离子源温度:200℃;
c(3 min) :
载氢气,纯度大于等于9.99为流1.0mL/ming)进样方式:无分流,1.0mm后开:矫剂延迟:2.8min
逃样量:2
电子轰击源EI能量:70e
监测方式:选择离子监测款式(STAI):k)
1)选择离子(m/2)定量2%,短性142、2真237:2%76.3.2气相色谱-质谱测定
根据样液中被测物含盐情况遥定峰面积棉运的运推工作潜没,标能工作溶液和待测样液中五筑硝基苯的响应值均应在仪器检测的我性范海内对标准工作游液与样液等体积参插进样测定。如架样液与标准工作溶液的趣择离子色谱图功,在相同探留时间有色谱峰出现,同时所选择离子的丰度比与标准样品相关离子的拍对率度一致(mz142、214、毫37、295元297,其丰度比100:73:85:51!36),相似度在允差之内(见表1).根据送凝离子m/z295对就进行外标法定量。在上述气相色谱-质谱条件下,五氮硝基苯的参考保留时间约为8.0min。五氯硝基苯标准品总离子流图和质谱图参见附录A中图A,1和图 A.2.
表1定性确证时相对离子度的最大充许偏差相对离了度/§
允许的相对确差/%
6. 4 空白试验
除不加试样外,均按上述测定步骤进行。7结果计算和表述
>10~-20
用色谱数据处理机或按式(1)计算试样中五氧硝基举残留量,计算结果应将空白值扣除:2
式中:
试样中五氧硝基苯的残留量,单位为毫克每干克(mg/kg):样被中五氧硝基苯的峰面积;
标推工作被中五氣硝基苯的峰面积:标准工作溶液中五氮硝基苯的度,单位为微克持毫升(rg/mL);样被最终定容体积,单位为升(mI):最终样液所代表的试样量,单位为克(g)。8方法的测定低限.回收率
8.1测定低限
本方法测定低限为:0.001mgkg.8.2回收率
本方法添加浓度范围及收率见装表2样品的添加浓度及回收率的实验数据样品
添加依度/(μg/kg)
回收率范}
25:- 954 0
20: 091, 0
25,c~1c0, 0
添加浓度/(rg/kg)
SN/r1957-2007
回收率范围/%
65. 0--97, 5
88,0--97.0
90. 3-- 97. 0
SN/T 1957-2007
附录A
(资料性附录)
五氮硝基苯标准品气相色谱-质图Maui
RT:2.76-12.94
4. M4 n Fr6 Dee. 527. 12
data 0107
022511155
五氨硝基苯标准品总离子流图(IIC)pn1_07U20152503F1034 RT:B.02 AV:1 SB:81B R.21 1D. 91,.:15-6.77 NI.12. 97E6T: (0,0]+= E2det-6D, 0 Full ms[85. 00-31 , 0]100
BIOEEEE
15.毫47.0
.182.8 207.8
五氛硝基苯标准品质谱图
.250. 8
462.8266.8
Foreword
Annex A of this standard is an informative annex.SN/I1957--2007
This standard was proposed by and is under the charge of China National Regulatory Commission forcertificationandAccreditation.This standard was drafted by tianjin entry-exit inspectian and quarantine bureau of the Peaple's re-pubic of China.
The main drafters of this standard are Lin Anging,Xu Hong,Zhang Man,Gu Long,He Jia,Zhang Jun,Wu Yanhui and Xiao Yabing.
This standard is a professional standard promulgated for the first time.SN/T1957—2007
Determination of quintozene resicues in medicinal plant andtheir products for import and export -Gc-Ms methodScope
This standard specifies the methods of determination by Gc-Ms of quintaze residue in medicinalplants and theirs products.
This standard js applicable to the peterminstion of quintoze residue(n panax,gen-seng.
2Principle method of determinationThe Quintozene residue were extractedrwith'acetong-n-hexane,sulfonaped by Oil of vitriol,determi-nated by GC-Ms. Calculated by conyparing peak at.3 Reagents and materials
Unless otherwise specified,all reagentswater.
n-Hexane; HPLC grade.
Acetone: HPLC grade.
Oil of vitriol,Guaranteed r
he sanple.withrcorresponding standard peak area.: :
hofa be anatyticaifx pure. \Water\is the first-degreeAnhydrous sodium sulfate:ignite at sso'c for 4 h,antr'store in air-tight container.Anhydrous sodium sulfate solution:40 g/LQuintozenestandard(C,Cl,NO2,CAS-No82-68-8).Purity99%Quintozene standard solution:Weigh appropriate quintozene,dissolve in n-Hexane and preparea solution of 10o μg/mL as standard stock solution. Dilute the quintozene standard stock solution tothe required concentration as the standard working sotution with n-Hexane. It should be stored in re-frigeratory at o'℃ ~4'℃.
SN/T 1957—2007
3, 8 Anhydrous sodium sulfate colurnn:80 mm × 40 mm(i. d. >funnel, filled with 10 anhydrous so-dium sulfate(3. 4) upon 5 mm.Apparatus and equipment
GC-MS: Equipped with El.
Minishaker
Homogenizer: 24 000 r/mil
Vortex mixer: 3 Do0 r/mir
Rotation evaporator.
Puiverizerbzxz.net
5 Sample preparation and storage Reduce the sample of panax to ca 500 g, churn up, let-pass- through a 2, 0 mm sieve, Reduce the sam-pleof gen-sengtoce5oog.Mixthoroughly,helow 4℃
Place in clean
containers, seal and label, storedIn the course ot sample preparafion, psecautidn should be taken to aviod contamination or any fac-tors which may cause the change Df qtintozene residie conteht.6 Procedure of determination6.1Extraction
6. 1.1 Panax sample
Weigh 5 gt accurated to 0. 0t g) of the test sanmple into 100 mL centrifuge tuba, adding 50 mL ace-tone-n-hexane (2 +B, V/ V), homogenize for 5 min under 14 0o0 r/min, centrifuge for 3 min under3 000 r/min. The extraction liquid was filterated to 150 ml erlenrneyer flake, ther repeated aboveaperation. Combine the extraction liquid, rotary evaporate the extraction liquid at 5o'C to almost50 ml. Transfer above solution to 150 mL separator funinel.SN/T 1957-2007
6. 1.2 Gen-seng sample
Weigh 5 g(accurated to 0.01 g) of the test sample into 100 mL centrifuge tube. adding 50 mL ace.tona-n-hexane.2+ 8, V/ V),minishaking 5 rnin, Transfer above solution to 500 mL separator funnel.adding 3o0 mL water, shaking.iet it stand for separately comperately. Abandon the awater phase.Adding 3oo mL water ,then repeated above operation.6.2Clean up
Add10m oil af vitriol (3.3),shaking lightly for 0,5min,Let it stand for separately comperatelyabandon the acid phase, Repeat above cleaning three or four times(until the acid phase show achro-maticity), Add 2 × 100 mL anhydrous sodizirn suifate solution (3. 5). let it stand for separately comp-erately, abandon the water phase. Pass this liquid through the column of anhydrous sodium suifate(3, 8) to another 150 mL erlenmeyer flake. Rinsc the cloumn of anhydrous sodium sulfate three timeswith 10 ml n-Hexan. Collect n-Hexane phase,rotary evaporate the extraction liquid at 5o' to dry.Make up to 1. 0 mL with n-hexane for GC-MS determination.6.3Determination
6. 3. 1Gc-Ms operating conditionsColumn; DB-35Ms 25 m × o, 25 mmci, d. >. film thickness 0. 25 μm ar equivalent+a)
10c/min-240c(3min));
Column temperature: t0a/75℃(3 min)b)
Injection port temperature: 28o'℃ :d)Interface tempcrature:250'C;e) lon soutce temperature:200' :f)Carrier gas:Helium,purity ≥99. 999%,flow rate;1.0 mL/min?g) Injection mode:Splitless, open the valve after 1. 0 min;Solvent protection delay:2. 8 minth)
i)Injection volumn:2 μL;
j)Jonizatian energY:70 evi
Detectian mode:SiM:
I)Sclected ions(m/z):determinded by295.confirmed by 142.214.237.297.6.3.2Gc-MS determination
SV/T 1957—2007
According to the approximate concentration of quintozene residuces in sample solution, select thestandard working salution with similar peak area to that af the sampla solution. The standard work-ing solutiori should be randomly injected in between the injaction of sarnple solution of equal volumn.if there is a peak appeared at the same retenticn time for both of the sample solution and standardworking solution in the mass spectrogram figure. The accordance between the retention time of themeasured sample solution and the timc of standard,the appearance of all selected ians in the chro-matogram of the sarnple while background is deducted. the consistency between the abundance ratioof the selected ions from the sample and ions from standard
6. 4 Blank test
20~50
Perfore the blank test with the same procederes as that described in the method of determination butwithout addition of test sample.7 Calculation and expression of the resuitsThe calculation of
A-the peak area of cuintozene of the sample solution:As--the peak area of quintozene the Standard working solution;Cs-the coricentration of quintozene in the Standard warking solution, μg/mL;V-the final volume of the sampie solution.ml:.(1)
SN/T 1957-2007
m-the massof test samplein the final solution,g.8Limit of determination and recovery8. 1Limit of determination
The limit of determination of this method is 0, 004 mg/kg.B.2Recovery
The range of fortification and recovery ot this method is shown in table 2Table 2The fortifying concenfratian of chiorfenapyr in samples and its corresponding recoveriesSampte
Fortify cncentration/
<μg/kg)
Retovery/%
75. 0~25, 0
80. D --94. 0
-85:0~100.0
Sampla
gen-seng
Fortlfy cncantration/
《μg/kg)
Recoverv/%
85. 0 ~97. 5
88.0~-97.0
90. 3~ 97. 0
小提示:此标准内容仅展示完整标准里的部分截取内容,若需要完整标准请到上方自行免费下载完整标准文档。
标准图片预览:




- 热门标准
- 商检行业标准(SN)
- SN/T1509-2005 异尖线虫病诊断规程
- SN/T1395.2-2005 禽衣原体病琼脂免疫扩散试验操作规程
- SN/T0328-94 出口氟石中氟化钙的化学分析方法
- SN/T3079.1-2012 进出口安全技术防范产品检验规程第1部分:安全防范报警设备
- SN/T1083.1-2002 焦炭分析试样水分、灰分的快速测定
- SN029-93 出口水果中双甲脒残留量检验方法
- SN/T4381-2015 食品接触材料纸、再生纤维材料使用改性聚苯醚测定纸和纸板迁移物的试验方法
- SN/T1443.2-2004 食品安全管理体系 审核指南
- SN0665-1997 出口肉及肉制品中雌三醇残留量检验方法放射免疫法
- SN0530-1996 出口肉中呋喃唑酮残留量的检验方法液相色谱法
- SN/T0380-1995 出口活鱼检验规程
- SN/T0877-2000 进出口发菜检验规程
- SN/T0794-1999 进出口西洋参检验规程
- SN/T0772-1999 出口真空软包装云腿片检验规程
- SN/T0801.6-1999 进出口动植物油脂沉积物检验方法
- 行业新闻
请牢记:“bzxz.net”即是“标准下载”四个汉字汉语拼音首字母与国际顶级域名“.net”的组合。 ©2025 标准下载网 www.bzxz.net 本站邮件:bzxznet@163.com
网站备案号:湘ICP备2025141790号-2
网站备案号:湘ICP备2025141790号-2