- 您的位置:
- 标准下载网 >>
- 标准分类 >>
- 商检行业标准(SN) >>
- SN/T 2290-2009 进出口化妆品中乙酰水杨酸的检测方法
标准号:
SN/T 2290-2009
标准名称:
进出口化妆品中乙酰水杨酸的检测方法
标准类别:
商检行业标准(SN)
标准状态:
现行-
发布日期:
2009-02-20 -
实施日期:
2009-09-01 出版语种:
简体中文下载格式:
.rar .pdf下载大小:
953.91 KB
部分标准内容:
中华人民共和国出入境检验检疫行业标准SN/T2290—2009
进出口化妆品中乙酰水杨酸的检测方法Determination of acetylsalicylic acid in cosmetics for import and export2009-02-20发布
中华人民共和国
国家质量监督检验检疫总局
2009-09-01实施
本标准的附录A、附录B和附录C均为资料性附录。本标准由国家认证认可监督管理委员会提出并归口。本标准起草单位:中华人民共和国广东出入境检验检疫局。本标准主要起草人:陈捷、奚星林、焦红、吴映、李荀本标准系首次发布的出入境检验检疫行业标准SN/T2290—2009
1范围
进出口化妆品中乙酰水杨酸的检测方法SN/T2290—2009
本标准规定了进出口化妆品中乙酰水杨酸高效液相色谱测定方法和液相色谱-质谱/质谱确证方法。本标准适用于美白、保湿等用途的面部使用的膏剂、乳霜和化妆水类化妆品中乙酰水杨酸的测定和确证。
2原理
用甲醇提取化妆品中的乙酰水杨酸,提取液经离心过滤后,用高效液相色谱法进行测定。外标法定量,液相色谱-质谱/质谱法确证。3试剂和材料
除非另有说明,水为二次去离子水或重蒸馏。3.1甲醇:色谱级。
3.2乙酸铵:分析醇。
3.3乙酸铵溶液(0.02mol/L):准确称取乙酸铵1.544g,用水溶解定容到1L。3.4乙酰水杨酸标准物质:纯度大于等于99%3.5乙酰水杨酸标准储备溶液:准确称取适量乙酰水杨酸标准物质(精确到0.1mg),以甲醇配制成浓度为1000mg/L的标准储备溶液。根据需要用甲醇稀释成适用浓度的标准工作溶液3.6乙酰水杨酸标准工作溶液:吸取一定量的标准储备液,用甲醇稀释成适当浓度的标准工作液.临用时现配。
4仪器与设备
高效液相色谱仪:配有紫外检测器液相色谱-质谱/质谱仪。
4.3分析天平:感量0.1mg。
4.4振荡器。
超声波清洗器
低温离心机:10000r/min。
聚丙烯塑料刻度离心管:50mL,具塞。滤膜:0.45um,有机系。
5测定步骤
5.1试样处理
称取化妆品试样约1g(精确到0.01g),置于50mL刻度离心管中,加入甲醇至10mL,在振荡机上振荡1min,超声波清洗器超声提取10min,在冰浴中放置10min,在5℃下,10000r/min离心10min,上清液经滤膜过滤,供高效液相色谱测定5.2测定
5.2.1色谱条件免费标准下载网bzxz
a)紫外检测器:波长230nm;
SN/T2290—2009
色谱柱:C18柱,250mmX4.6mm(内径),5μm,或相当者;b)
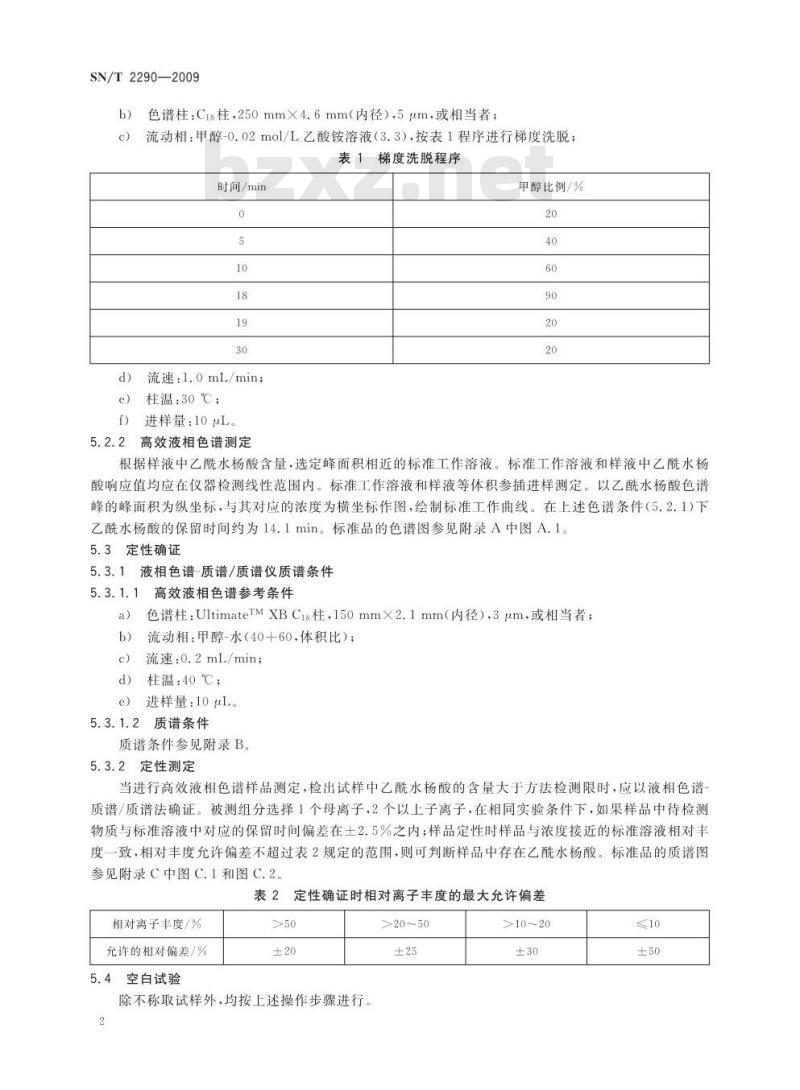
流动相:甲醇-0.02mo1/L乙酸铵溶液(3.3),按表1程序进行梯度洗脱:表1梯度洗脱程序
时间/min
流速:1.0mL/min;
柱温:30℃;
进样量:10μL。
5.2.2高效液相色谱测定
甲醇比例/%
根据样液中乙酰水杨酸含量,选定峰面积相近的标准工作溶液。标准工作溶液和样液中乙水杨酸响应值均应在仪器检测线性范围内。标准工作溶液和样液等体积参插进样测定。以乙酰水杨酸色谱峰的峰面积为纵坐标,与其对应的浓度为横坐标作图,绘制标准工作曲线。在上述色谱条件(5.2.1)下乙酰水杨酸的保留时间约为14.1min。标准品的色谱图参见附录A中图A.1。5.3定性确证
5.3.1液相色谱-质谱/质谱仪质谱条件高效液相色谱参考条件
色谱柱:UltimateTMXBCig柱,150mmX2.1mm(内径).3μm,或相当者;流动相:甲醇-水(40+60.体积比);流速:0.2mL/min;
柱温:40℃;
进样量:10μL。
5.3.1.2质谱条件
质谱条件参见附录B。
5.3.2定性测定
当进行高效液相色谱样品测定,检出试样中乙酰水杨酸的含量大于方法检测限时,应以液相色谱质谱/质谱法确证。被测组分选择1个母离子,2个以上子离子,在相同实验条件下,如果样品中待检测物质与标准溶液中对应的保留时间偏差在土2.5%之内;样品定性时样品与浓度接近的标准溶液相对丰度一致,相对丰度充许偏差不超过表2规定的范围,则可判断样品中存在乙酰水杨酸。标准品的质谱图参见附录C中图C.1和图C.2。
表2定性确证时相对离子丰度的最大允许偏差相对离子丰度/%
允许的相对偏差/%
5.4空白试验
除不称取试样外,均按上述操作步骤进行。2
>20~50
>10~20
结果计算
SN/T 2290—2009
用色谱数据处理机或按(1)式计算试样中乙酰水杨酸的含量,计算结果需扣除空白值exV
式中:
-试样中乙酰水杨酸含量,单位为毫克每千克(mg/kg);.....
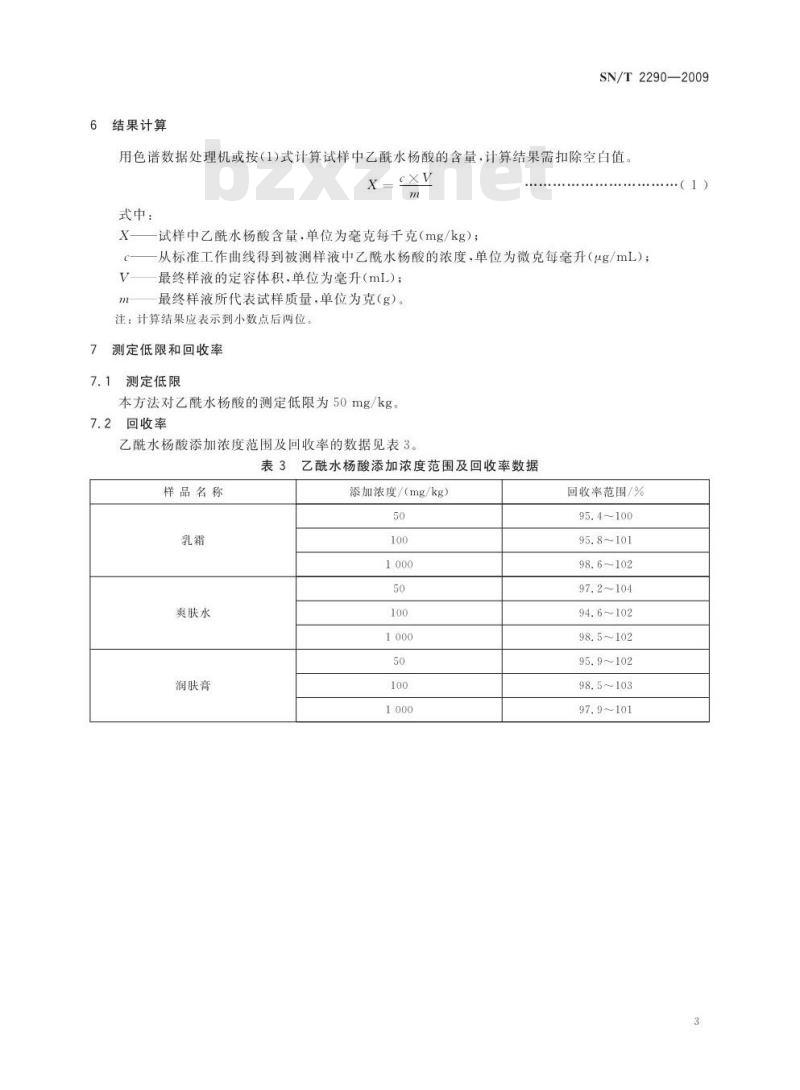
从标准工作曲线得到被测样液中乙酰水杨酸的浓度,单位为微克每毫升(ug/mL);最终样液的定容体积,单位为毫升(mL);最终样液所代表试样质量,单位为克(g)。注:计算结果应表示到小数点后两位。测定低限和回收率
测定低限
本方法对乙酰水杨酸的测定低限为50mg/kg。回收率
乙酰水杨酸添加浓度范围及回收率的数据见表3。表3乙酰水杨酸添加浓度范围及回收率数据样品名称
爽肤水
润肤膏
添加浓度/(mg/kg)
回收率范围/%
SN/T2290—2009
nV 2 500.04
2 000.00E
附录A
(资料性附录)
乙酰水杨酸标准品色谱图
6. 008. 00t0. 00 12. 00 74. 00 16. 00 18. 00 20. 08 t/min0. 002. 04. 00
1乙酰水杨酸。
质谱参数
雾化气
气帘气
辅助加热气/(L/min)
碰撞气
辅助加热气温度/℃
喷雾电压/V
去籁电压/V
碰撞能/V
采集时间/ms
乙酸水杨酸标准品(5μg/mL)色谱图附录B
(资料性附录)
参考质谱参数1)
参考质谱参数
参数值
11(m/z179.1/136.8),-33(m/z179.1/93.2)100
非商业性声明,质谱条件是在API3000液相色谱-质谱/质谱联用仪上完成,此处列出试验用仪器型号仅为提供参考,并不涉及商业目的·鼓励标准使用者尝试不同厂家或型号的仪器。7. 0e5
. tie4
附录C
(资料性附录)
乙酰水杨酸标准物质LC/MS/MS质谱图和色谱图136. A
179.1/136.8
(m/x/amu
乙酰水杨酸标准品离子全扫描质谱图2. 04h
6 000. 0F
SN/T 2290—2009
179.1/93.2
图C.2乙酰水杨酸标准品多反应监测(MRM)色谱图180
10t/min
SN/T2290—2009
Foreword
AnnexAand Annex B and AnnexC of this standard arean informative annexes.This standard was proposed by and is under the jurisdiction of the Certification and AccreditationAdministrationof thePeople'sRepublicofChina.This standard is drafted by Chinese Academy of Inspection and Quarantine.Maindraftersof thisstandardare:ChengJie,XiXinglin,JiaoHong,WuYingxuan,LiXun.This standard is an inspection and quarantine professional standard of the People's Republic of Chinapromulgated forthefirsttime.Note:This English Version.atranslation from the Chinese text,is solely for guidance6
SN/T 2290—2009
Determination of acetylsalicylic acid in cosmeticsfor import and export
This standard specifies the method of determination of acetylsalicylic acid in cosmetics for importandexportbyHPLCandHPLC-MS/MSThis standard is applicable to the determination of acetylsalicylic acid in cosmetics for cream andpleasium refreshing toner and lotion.2Principle
Acetylsalicylic acid is extracted from sample with methanol. After centrifugation of extraction sol-vents,acetylsalicylic acid isdeterminated by HPLC and quantified by external standard method.Ace-tylsalicylicacid is confirmedbyHPLC-MS/MS3
Reagentsandmaterials
Unless otherwise specified,all regents used are A. R.,and pure \water\ is redistilled water3.1
Methanol:HPLCgrade
ammoniumacetate:A.R.
0.02 mol/L ammonium acetate: Dilute 1.544 g ammonium acetate(3.2)in 1 L waterStandard:Acetylsalicylicacid,purity≥99%Standard stock solution:Accurately weigh an adequate amount of each standard(accurate to3.5
0.1mg),dissolve inmethanol and preparea solution of 1ooomg/Lasthe standard stock solution.Standard working solution:According to the requirement,dilute the standard solution to ap-3.6
propriateconcentrationwithmethanol justbeforeuse.4Apparatusandequipment
4.1LiquidchromatographyequippedwithUVdetectorSN/T2290—2009
LiquidChromatograph-MassSpectrometryBalance:Accuracy:0.1mg
Vortexmixer.
Ulitrasonic waterbath.
Lowtemperature centrifuge:10 000 r/min.Centrifugetube:polytetrafluoroethylene.5omL.filtermembrane:0.45μm,organicsystem.Procedure
Preparationoftestsamples
Weigh about 1 g(accurate to 0.01 g)of the test sample into a 50 mL polytetrafluoroethylene centri-fugetube,add10mLofmethanoltothetube.Shakethetubefor1minbyvortexmixer,extract inultrason-ic water bath for 10 min,then place the tube in ice bath for 10 min. Centrifuge at 10 000 r/min for 10 min.FilterthesolutionintoaHPLCvialthroughao.45μmsyringefilter.5.2
Determination
HPLC operating conditions
UVdetector:wave230nm;
Column:C18.250mmx4.6mm(i.d.),5μm.orequivalentMobilephase:methanol-o.02mol/Lammoniumacetate,gradientseetable1;Table 1-The mobile phase wash gradient of HPLCTime/min
Methanol/%
Flowrate:1.0mL/min;
Column thermostat:30 ℃;
Injectionvolume:10μL.
HPLCdetermination
SN/T2290—2009
According to the estimated approximate concentration of acetylsalicylic acid in the sample solution,selectthe standard working solution of similar concentration to that of sample solution.The respon-ses of acetylsalicylic acid in the sample solution should be in the linear range of the instrumental de-tection.Thestandard working solution should beinjected randomly in betweenthe injections of thesample of equal volume. Under the above instrumental condition,the retention time of acetylsalicylicacid is 14. 1 min,For the chromatogram of acetylsalicylic acid standard,see annex A,Figure A. 1.5.3
Confirmation
5.3.1Ms/Msoperatingconditions5.3.1.1HPLCoperatingconditionsa)
LCcolumn:UltimateTMXBC8,150mmx2.1mm(i.d.).3μmorequivalent;Mobilephase:methanol-water(40+60.V/V);Flow rate: 0.2 mL/min;
Columnthermostat:40℃;
Injection volume:10 μL
Ms/Msoperatingconditions
Ms/Ms operating conditions seeannexB5.3.2Confirmation procedure
Whenthecontentof acetylsalicylic acid intest sampledeterminedbyHPLc isgreaterthan limit ofmethod,confirmation should becarried out byMs/Ms.A Precursor ion and two daughterions arechosen.Underabovedetermination condition,the variation range of the retentiontimefor the peakof analyteinunknown sampleandinthe standard working solution can notbe out of rangeof±2.5%. For the same analysis batch and the same compound,the variation range of the ion ratio be-tweenthetwodaughterionsfortheunknownsampleandthestandardworkingsolution atthesimilar9
SN/T2290—2009
concentration can not be out of range of table 2,and then the corresponding analyte must be presentin the sample.MRM Chromatogram of the standard seeannex C,Figure C.1 and Figure C.2.Table2-Maximum permitted tolerances for relative ion intensities while confirmationRelative intensity/%
Permittedtolerances/%
Blank test
>20~50
>10~20
Theoperationof blanktestisthesameasabove,exceptwithoutsample.6
Calculationandexpresssionofresult10
Thecontentof acetylsalicylic acid is calculated by computer processororbythefollowing formula(1).Theblankvalueshouldbesubtractedfromtheresultof calculation.cxV
X-thecontentofacetylsalicylicacidintestsample.mg/kgc-theconcentration inthestandardworking solution,μg/mLV-thefinal volumeof sample solution,mL;m-thecorrespondingmassoftestsampleinthefinal samplesolution,gNote: show two decimal places in expresssion of result.Limitof determinationand recovery7.1 Limit of determination
The limit of determination of this method is 50 mg/kg.7.2Recovery
Recoveryof acetylsalicylic acidseetable3.10
.(1)
小提示:此标准内容仅展示完整标准里的部分截取内容,若需要完整标准请到上方自行免费下载完整标准文档。
进出口化妆品中乙酰水杨酸的检测方法Determination of acetylsalicylic acid in cosmetics for import and export2009-02-20发布
中华人民共和国
国家质量监督检验检疫总局
2009-09-01实施
本标准的附录A、附录B和附录C均为资料性附录。本标准由国家认证认可监督管理委员会提出并归口。本标准起草单位:中华人民共和国广东出入境检验检疫局。本标准主要起草人:陈捷、奚星林、焦红、吴映、李荀本标准系首次发布的出入境检验检疫行业标准SN/T2290—2009
1范围
进出口化妆品中乙酰水杨酸的检测方法SN/T2290—2009
本标准规定了进出口化妆品中乙酰水杨酸高效液相色谱测定方法和液相色谱-质谱/质谱确证方法。本标准适用于美白、保湿等用途的面部使用的膏剂、乳霜和化妆水类化妆品中乙酰水杨酸的测定和确证。
2原理
用甲醇提取化妆品中的乙酰水杨酸,提取液经离心过滤后,用高效液相色谱法进行测定。外标法定量,液相色谱-质谱/质谱法确证。3试剂和材料
除非另有说明,水为二次去离子水或重蒸馏。3.1甲醇:色谱级。
3.2乙酸铵:分析醇。
3.3乙酸铵溶液(0.02mol/L):准确称取乙酸铵1.544g,用水溶解定容到1L。3.4乙酰水杨酸标准物质:纯度大于等于99%3.5乙酰水杨酸标准储备溶液:准确称取适量乙酰水杨酸标准物质(精确到0.1mg),以甲醇配制成浓度为1000mg/L的标准储备溶液。根据需要用甲醇稀释成适用浓度的标准工作溶液3.6乙酰水杨酸标准工作溶液:吸取一定量的标准储备液,用甲醇稀释成适当浓度的标准工作液.临用时现配。
4仪器与设备
高效液相色谱仪:配有紫外检测器液相色谱-质谱/质谱仪。
4.3分析天平:感量0.1mg。
4.4振荡器。
超声波清洗器
低温离心机:10000r/min。
聚丙烯塑料刻度离心管:50mL,具塞。滤膜:0.45um,有机系。
5测定步骤
5.1试样处理
称取化妆品试样约1g(精确到0.01g),置于50mL刻度离心管中,加入甲醇至10mL,在振荡机上振荡1min,超声波清洗器超声提取10min,在冰浴中放置10min,在5℃下,10000r/min离心10min,上清液经滤膜过滤,供高效液相色谱测定5.2测定
5.2.1色谱条件免费标准下载网bzxz
a)紫外检测器:波长230nm;
SN/T2290—2009
色谱柱:C18柱,250mmX4.6mm(内径),5μm,或相当者;b)
流动相:甲醇-0.02mo1/L乙酸铵溶液(3.3),按表1程序进行梯度洗脱:表1梯度洗脱程序
时间/min
流速:1.0mL/min;
柱温:30℃;
进样量:10μL。
5.2.2高效液相色谱测定
甲醇比例/%
根据样液中乙酰水杨酸含量,选定峰面积相近的标准工作溶液。标准工作溶液和样液中乙水杨酸响应值均应在仪器检测线性范围内。标准工作溶液和样液等体积参插进样测定。以乙酰水杨酸色谱峰的峰面积为纵坐标,与其对应的浓度为横坐标作图,绘制标准工作曲线。在上述色谱条件(5.2.1)下乙酰水杨酸的保留时间约为14.1min。标准品的色谱图参见附录A中图A.1。5.3定性确证
5.3.1液相色谱-质谱/质谱仪质谱条件高效液相色谱参考条件
色谱柱:UltimateTMXBCig柱,150mmX2.1mm(内径).3μm,或相当者;流动相:甲醇-水(40+60.体积比);流速:0.2mL/min;
柱温:40℃;
进样量:10μL。
5.3.1.2质谱条件
质谱条件参见附录B。
5.3.2定性测定
当进行高效液相色谱样品测定,检出试样中乙酰水杨酸的含量大于方法检测限时,应以液相色谱质谱/质谱法确证。被测组分选择1个母离子,2个以上子离子,在相同实验条件下,如果样品中待检测物质与标准溶液中对应的保留时间偏差在土2.5%之内;样品定性时样品与浓度接近的标准溶液相对丰度一致,相对丰度充许偏差不超过表2规定的范围,则可判断样品中存在乙酰水杨酸。标准品的质谱图参见附录C中图C.1和图C.2。
表2定性确证时相对离子丰度的最大允许偏差相对离子丰度/%
允许的相对偏差/%
5.4空白试验
除不称取试样外,均按上述操作步骤进行。2
>20~50
>10~20
结果计算
SN/T 2290—2009
用色谱数据处理机或按(1)式计算试样中乙酰水杨酸的含量,计算结果需扣除空白值exV
式中:
-试样中乙酰水杨酸含量,单位为毫克每千克(mg/kg);.....
从标准工作曲线得到被测样液中乙酰水杨酸的浓度,单位为微克每毫升(ug/mL);最终样液的定容体积,单位为毫升(mL);最终样液所代表试样质量,单位为克(g)。注:计算结果应表示到小数点后两位。测定低限和回收率
测定低限
本方法对乙酰水杨酸的测定低限为50mg/kg。回收率
乙酰水杨酸添加浓度范围及回收率的数据见表3。表3乙酰水杨酸添加浓度范围及回收率数据样品名称
爽肤水
润肤膏
添加浓度/(mg/kg)
回收率范围/%
SN/T2290—2009
nV 2 500.04
2 000.00E
附录A
(资料性附录)
乙酰水杨酸标准品色谱图
6. 008. 00t0. 00 12. 00 74. 00 16. 00 18. 00 20. 08 t/min0. 002. 04. 00
1乙酰水杨酸。
质谱参数
雾化气
气帘气
辅助加热气/(L/min)
碰撞气
辅助加热气温度/℃
喷雾电压/V
去籁电压/V
碰撞能/V
采集时间/ms
乙酸水杨酸标准品(5μg/mL)色谱图附录B
(资料性附录)
参考质谱参数1)
参考质谱参数
参数值
11(m/z179.1/136.8),-33(m/z179.1/93.2)100
非商业性声明,质谱条件是在API3000液相色谱-质谱/质谱联用仪上完成,此处列出试验用仪器型号仅为提供参考,并不涉及商业目的·鼓励标准使用者尝试不同厂家或型号的仪器。7. 0e5
. tie4
附录C
(资料性附录)
乙酰水杨酸标准物质LC/MS/MS质谱图和色谱图136. A
179.1/136.8
(m/x/amu
乙酰水杨酸标准品离子全扫描质谱图2. 04h
6 000. 0F
SN/T 2290—2009
179.1/93.2
图C.2乙酰水杨酸标准品多反应监测(MRM)色谱图180
10t/min
SN/T2290—2009
Foreword
AnnexAand Annex B and AnnexC of this standard arean informative annexes.This standard was proposed by and is under the jurisdiction of the Certification and AccreditationAdministrationof thePeople'sRepublicofChina.This standard is drafted by Chinese Academy of Inspection and Quarantine.Maindraftersof thisstandardare:ChengJie,XiXinglin,JiaoHong,WuYingxuan,LiXun.This standard is an inspection and quarantine professional standard of the People's Republic of Chinapromulgated forthefirsttime.Note:This English Version.atranslation from the Chinese text,is solely for guidance6
SN/T 2290—2009
Determination of acetylsalicylic acid in cosmeticsfor import and export
This standard specifies the method of determination of acetylsalicylic acid in cosmetics for importandexportbyHPLCandHPLC-MS/MSThis standard is applicable to the determination of acetylsalicylic acid in cosmetics for cream andpleasium refreshing toner and lotion.2Principle
Acetylsalicylic acid is extracted from sample with methanol. After centrifugation of extraction sol-vents,acetylsalicylic acid isdeterminated by HPLC and quantified by external standard method.Ace-tylsalicylicacid is confirmedbyHPLC-MS/MS3
Reagentsandmaterials
Unless otherwise specified,all regents used are A. R.,and pure \water\ is redistilled water3.1
Methanol:HPLCgrade
ammoniumacetate:A.R.
0.02 mol/L ammonium acetate: Dilute 1.544 g ammonium acetate(3.2)in 1 L waterStandard:Acetylsalicylicacid,purity≥99%Standard stock solution:Accurately weigh an adequate amount of each standard(accurate to3.5
0.1mg),dissolve inmethanol and preparea solution of 1ooomg/Lasthe standard stock solution.Standard working solution:According to the requirement,dilute the standard solution to ap-3.6
propriateconcentrationwithmethanol justbeforeuse.4Apparatusandequipment
4.1LiquidchromatographyequippedwithUVdetectorSN/T2290—2009
LiquidChromatograph-MassSpectrometryBalance:Accuracy:0.1mg
Vortexmixer.
Ulitrasonic waterbath.
Lowtemperature centrifuge:10 000 r/min.Centrifugetube:polytetrafluoroethylene.5omL.filtermembrane:0.45μm,organicsystem.Procedure
Preparationoftestsamples
Weigh about 1 g(accurate to 0.01 g)of the test sample into a 50 mL polytetrafluoroethylene centri-fugetube,add10mLofmethanoltothetube.Shakethetubefor1minbyvortexmixer,extract inultrason-ic water bath for 10 min,then place the tube in ice bath for 10 min. Centrifuge at 10 000 r/min for 10 min.FilterthesolutionintoaHPLCvialthroughao.45μmsyringefilter.5.2
Determination
HPLC operating conditions
UVdetector:wave230nm;
Column:C18.250mmx4.6mm(i.d.),5μm.orequivalentMobilephase:methanol-o.02mol/Lammoniumacetate,gradientseetable1;Table 1-The mobile phase wash gradient of HPLCTime/min
Methanol/%
Flowrate:1.0mL/min;
Column thermostat:30 ℃;
Injectionvolume:10μL.
HPLCdetermination
SN/T2290—2009
According to the estimated approximate concentration of acetylsalicylic acid in the sample solution,selectthe standard working solution of similar concentration to that of sample solution.The respon-ses of acetylsalicylic acid in the sample solution should be in the linear range of the instrumental de-tection.Thestandard working solution should beinjected randomly in betweenthe injections of thesample of equal volume. Under the above instrumental condition,the retention time of acetylsalicylicacid is 14. 1 min,For the chromatogram of acetylsalicylic acid standard,see annex A,Figure A. 1.5.3
Confirmation
5.3.1Ms/Msoperatingconditions5.3.1.1HPLCoperatingconditionsa)
LCcolumn:UltimateTMXBC8,150mmx2.1mm(i.d.).3μmorequivalent;Mobilephase:methanol-water(40+60.V/V);Flow rate: 0.2 mL/min;
Columnthermostat:40℃;
Injection volume:10 μL
Ms/Msoperatingconditions
Ms/Ms operating conditions seeannexB5.3.2Confirmation procedure
Whenthecontentof acetylsalicylic acid intest sampledeterminedbyHPLc isgreaterthan limit ofmethod,confirmation should becarried out byMs/Ms.A Precursor ion and two daughterions arechosen.Underabovedetermination condition,the variation range of the retentiontimefor the peakof analyteinunknown sampleandinthe standard working solution can notbe out of rangeof±2.5%. For the same analysis batch and the same compound,the variation range of the ion ratio be-tweenthetwodaughterionsfortheunknownsampleandthestandardworkingsolution atthesimilar9
SN/T2290—2009
concentration can not be out of range of table 2,and then the corresponding analyte must be presentin the sample.MRM Chromatogram of the standard seeannex C,Figure C.1 and Figure C.2.Table2-Maximum permitted tolerances for relative ion intensities while confirmationRelative intensity/%
Permittedtolerances/%
Blank test
>20~50
>10~20
Theoperationof blanktestisthesameasabove,exceptwithoutsample.6
Calculationandexpresssionofresult10
Thecontentof acetylsalicylic acid is calculated by computer processororbythefollowing formula(1).Theblankvalueshouldbesubtractedfromtheresultof calculation.cxV
X-thecontentofacetylsalicylicacidintestsample.mg/kgc-theconcentration inthestandardworking solution,μg/mLV-thefinal volumeof sample solution,mL;m-thecorrespondingmassoftestsampleinthefinal samplesolution,gNote: show two decimal places in expresssion of result.Limitof determinationand recovery7.1 Limit of determination
The limit of determination of this method is 50 mg/kg.7.2Recovery
Recoveryof acetylsalicylic acidseetable3.10
.(1)
小提示:此标准内容仅展示完整标准里的部分截取内容,若需要完整标准请到上方自行免费下载完整标准文档。
标准图片预览:



- 热门标准
- 商检行业标准(SN)
- SN/T1509-2005 异尖线虫病诊断规程
- SN/T2558.10-2015 进出口纺织品功能性检测方法第10部分:吸水性
- SN0665-1997 出口肉及肉制品中雌三醇残留量检验方法放射免疫法
- SN/T1672.7-2013 进出口医用设备检验规程 第7部分:医用内窥镜
- SN0020-92 出口磁带收录音机安全要求检验规程
- SN/T0226.2-1993 出口冻公鱼检验规程
- SN/T0380-1995 出口活鱼检验规程
- SN/T1359.2-2004 进口纺织机械检验规程 织袜机
- SN/T1657.2-2005 进出口电动上具检验规程 第2部分:电链锯
- SN/T1395.2-2005 禽衣原体病琼脂免疫扩散试验操作规程
- SN/T3719.1-2013 进出口纺织行业成套设备检验技术要求 第1部分化纤设备
- SN/T0328-94 出口氟石中氟化钙的化学分析方法
- SN/T4569-2016 生物柴油中甲醇、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、叔丁醇及仲丁醇的测定气相色谱法
- SN/T2374-2009 绿僵菌、白僵菌生物农药检验操作规程
- SN/T4525.4-2016 出口食品中致病菌的分子分型MLST方法 第4部分:霍乱弧菌
- 行业新闻
请牢记:“bzxz.net”即是“标准下载”四个汉字汉语拼音首字母与国际顶级域名“.net”的组合。 ©2025 标准下载网 www.bzxz.net 本站邮件:bzxznet@163.com
网站备案号:湘ICP备2025141790号-2
网站备案号:湘ICP备2025141790号-2